| Research Article | ||
J. Microbiol. Infect. Dis., (2024), Vol. 14(2): 60–66 Research Article Pre-treatment HIV-1 drug resistance in Cuban patients with late presentation to health care: 2009–2020Liuber Y. Machado1*, Héctor M. Díaz2, Liodelvio Martínez2, Madeline Blanco1, Marta Dubed1, Neisy Valdés1, Karen Valdés2, Laura S. López1, Enrique Noa1, María T. Pérez1 Otto Cruz1 and Mireida Rodríguez11AIDS Research Laboratory, Mayabeque, Cuba 2Hermanos Ameijeiras Hospital, Havana, Cuba *Corresponding Author: Liuber Y. Machado. AIDS Research Laboratory, Mayabeque, Cuba. Email: liuberyans [at] infomed.sld.cu; liuberyans1980 [at] gmail.com Submitted: 12/03/2024 Accepted: 23/04/2024 Published: 30/06/2024 © 2024 Journal of Microbiology and Infectious Diseases
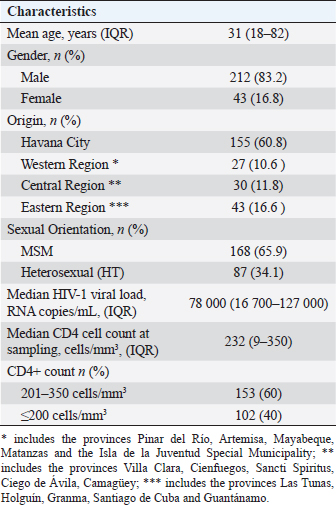
AbstractBackground: The presence of antiretroviral-resistant human immunodeficiency virus (HIV)-1 genetic variants in patients with late presentation to health care may impact the success of antiretroviral therapy (ART), costs of care, and potential HIV transmission. Aim: To determine the prevalence of pre-treatment HIV-1 drug resistance to antiretrovirals (pre-treatment HIV-1 drug resistance (PDR)) in Cuban patients classified as late presenters (LPs). Methods: A retrospective cross-sectional study was carried out on 255 HIV-positive Cubans classified as LP (CD4+ T cell count ≤ 350 cells/mm3) and included in PDR surveillance studies in Cuba during the period 2009–2020. The viral subtype and the genotypic profile of antiretroviral resistance were analyzed. Some clinical and epidemiological variables were evaluated. Results: 83.2% of the patients were male and 65.9% were men who had sex with other men. The average age was 31 years. 40% of the individuals studied had advanced disease (CD4+ T cell count ≤200 cells/mm3). The predominant genetic variants were subtype B (29.4%); CRF20, 23, 24_BG (24.3%), and CRF19_cpx (22.4%). The prevalence of pre-treatment HIV-1 drug resistance (PDR) was 21.2% (95%, CI 16.3–26.7). The prevalence was 8.2% (95%, CI 5.3–11.9) for any nucleoside reverse transcriptase inhibitor (NRTI), 15.1% (95%, CI 11.2–19.7) for any non- NRTI (NNRTI), and 2.1% (95%, CI 0.75–4.41) for any protease inhibitor (PI). 9.25% of the LPs with PDR died a year after diagnosis of the HIV infection. The most frequent mutations were K103N (6.7%), G190A (4.7%), and M184V (4.3%). The treatment will not be effective in 17.3% of patients who start therapy with the NRTI + NNRTI combinations. Conclusion: The high PDR values in LPs show the need to enhance genotypic studies of HIV resistance in this population at the time of diagnosis of the infection, since the success of first-line ART may be compromised and influence compliance with the global goals set forth by United nations acquired immunodeficiency syndrome for the year 2030. Keywords: Cuba, Late presenters, Resistance, Antiretroviral therapy, HIV. IntroductionThe use of antiretroviral therapy (ART) has reduced mortality and morbidity in people living with human immunodeficiency virus (HIV) (PLWH) (Günthard et al., 2014). However, these results largely depend on early diagnosis of HIV infection and early initiation of ART. The global quest to end the HIV/acquired immunodeficiency syndrome (AIDS) pandemic by 2030 has focused its strategies on diagnosing, treating, and maximizing viral suppression among PLWH, with the goal of preventing AIDS-related diseases and reducing the risk of HIV transmission (WHO, 2016). Late presentation to health care is one of the problems that can compromise the achievement of global goals. Late presenters (LPs), according to a consensus definition, are HIV-infected individuals who present with an initial CD4 cell count of less than 350 cells/mm3 or an AIDS-defining event, regardless of the CD4 cell count (Antinori et al., 2011). LPs are more likely to transmit HIV, are associated with a negative impact on mortality and morbidity, and increase health care costs, resulting from higher rates of hospitalization and treatment of opportunistic infections in addition to primary treatment of the HIV infection (Guaraldi et al., 2017). Transmitted and pre-treatment HIV drug resistance also constitutes a barrier to meeting the goals set forth by United nations acquired immunodeficiency syndrome (UNAIDS). The World Health Organization (WHO) has provided a global action plan on HIV drug resistance with the aim of minimizing the emergence of drug resistance, prolonging the effectiveness of first- and second-line therapies and improving the quality of life of PLWH (WHO, 2017). HIV drug resistance surveillance actions in the newly diagnosed population without ART, through national surveys or periodic studies, provide elements that allow the fulfillment of the objectives stated in said strategy. The presence of HIV variants with mutations associated with resistance in some of the drugs used in first-line ART regimens can compromise its success in PLWH (Tang and Shafer, 2012), and if is an LPs, the probabilities of failure and poor evolutionary prognosis will be greater. Although late diagnosis of HIV continues to be a problem in low- and middle-income countries (WHO, 2023), there are few studies on the behavior of HIV pre-treatment resistance in LPs. Within the premises of the National Strategy for the diagnosis of HIV in Cuba, there is broad population coverage and early diagnosis (Díaz et al., 2011). Until December 2023, data from the Cuban Ministry of Public Health report a cumulative number of 41,178 people diagnosed with HIV infection since 1986, of which 32,808 are alive, 27,739 PLWH receive ART, and of them, 24,771 (89.3%) achieved viral suppression (MINSAP Computerized Registry, 2023). In Cuba, between 9% and 15% of people who are diagnosed with HIV infection in a year, are classified as LPs (MINSAP Computerized Registry, 2023). Studying the behavior and factors that influence the late presentation of HIV constitutes a challenge for National Programs, which must explore strategies that reduce its appearance and thus achieve compliance with UNAIDS goals. The National sexual transmission infections/HIV/AIDS Program of Cuba has incorporated into the national strategic plan for the prevention and control of STIs, HIV, and viral hepatitis, a surveillance strategy for HIV drug resistance in the newly diagnosed population. The objective of the present study is to determine the prevalence of pre-treatment HIV-1 drug resistance (PDR) in Cuban PLWH classified as LPs. Materials and MethodsSample population and inclusion criteriaA descriptive and retrospective cross-sectional study was carried out on 255 Cuban PLWH classified as LPs (CD4 + T cell count ≤ 350 cells/mm3) in the period 2009–2020 and previously included in HIV-1 drug resistance surveillance studies in Cuba. Clinical, demographic, and epidemiological data were obtained after approval of the informed consent and before sample collection. LPs with CD4 cell count ≤200 cells/mm3 were classified as individuals with advanced disease (WHO, 2017). HIV-1 sequencing and evaluation of antiretroviral resistanceHIV RNA was extracted from 1 ml of plasma (QIAamp Viral RNA kit, QIAGEN, Valencia, CA). The protease (PR) and reverse transcriptase (RT) regions were amplified using an “in house” procedure (Quarleri et al., 2004; Alemán et al., 2015). The sequences were obtained using the GenomeLabTM DTCS Quickstar kit (Beckman Coulter, USA) on a CEQ TM 8800 genetic analyzer (Beckman Coulter, USA) and assembled using the Sequencher program version 5.0 (GeneCodes, Inc, Ann Arbor, MI). HIV-1 subtype was determined using the REGA HIV-1 subtypingtool v 3.0 program (http://dbpartners.stanford.edu:8080/RegaSubtyping/stanford-hiv/typingtool/) and confirmed by phylogenetic analysis. The presence of mutations associated with transmitted resistance was determined using the CPR tool v 5.0 (http://hivdb.stanford.edu) which uses the list of mutations from the transmitted resistance surveillance of 2009 (Bennet et al., 2009). Resistance levels were determined using the HIVdb tool version 8.2.0 of the HIV ARV resistance database of Stanford University (http://hivdb.stanford.edu). Resistance levels were classified according to the Stanford penalties (SS): high (≥60), intermediate (30–59), and low (15–29). To evaluate the quality control of the sequences, the WHO HIVDR tool program (http://pssm.cfenet.ubc.ca/who_qc) was used. Statistical analysisA database was created in Excel, where the characteristics of those involved in the study were described, according to the mean, standard deviation, median, interquartile range, and frequency (%). Comparisons between groups were determined using the Fisher or chi-square test for categorical variables and for prevalence values, the 95% confidence intervals were calculated using the modified Wald method. The GraphPad Prims 6 program (San Diego, CA) was used. Ethical approvalThe ethical procedures were carried out according to the requirements or standards of the Ministry of Public Health of the Republic of Cuba and the Ministry of Science, Technology, and Environment, which contemplates the principles enunciated in the Declaration of Helsinki. This study was conducted with ethical clearance by the Research on Human Subjects (Medical) Committee at the AIDS Research Laboratory, Cuba (CIE-LISIDA-16-04-1.2). Before sample collection, informed consent was obtained from each patient who participated in the study. ResultsThe median age was 31 years, with values ranging from 18 to 82 years. 83.2% of PLWH belonged to the male sex and 65.9% were grouped in the group with the sexual orientation of men who have sex with other men (MSM). The median viral load was 78,000 RNA copies/mL and the CD4+ cell count was 232 cells/mm3 (Table 1). 40% of the patients studied had a CD4+ cell count below 200 cells/mm3, a value that classifies them as having advanced disease (Table 1). Subtype B (29.4%) predominated in the samples studied, followed by CRF20, 23, 24_BG (24.3%), CRF19_cpx (22.4%), CRF18_cpx (10.6%), unique recombinant form (URF) (9.4%) and other subtypes (3.9%). The prevalence of pre-treatment HIV-1 drug resistance in the LP studied for any antiretroviral was 21.2% (95% CI, 16.3–26.7), with a predominance in the group of drugs corresponding to the non-nucleoside RT inhibitor (NNRTIs) (15.1%; CI 95% 11.2–19.7). 1.02% of LPs presented resistance to the three families of antiretrovirals (Table 2). The most frequent mutations associated with resistance to NNRTIs were K103N (6.7%); Y181C (4.37%); G190A (3.14%). M184V/I associated with resistance to nucleoside RT inhibitors (NRTIs) was detected in 4.3% of the samples studied and D30N, associated with resistance to PR inhibitors (PIs), was identified in 1.17%. LPs with a CD4+ count below 200 cells/mm3 had a higher prevalence of pre-treatment HIV-1 drug resistance (27.4%; 95% CI, 17.7–35.7). There were no significant differences when comparing pre-treatment HIV drug resistance values with gender and sexual orientation. URF were the genetic variants with the highest prevalence of pre-treatment HIV-1 drug resistance (29.2%, 95% CI, 12.6–51.1), followed by CRF19_cpx (22.8%; 95% CI, 12.7–35.8), mainly due to the NNRTI family. Table 1. Clinical and epidemiological characteristics of the 255 PLWH-1 classified as LPs included in the study (2009–2020).
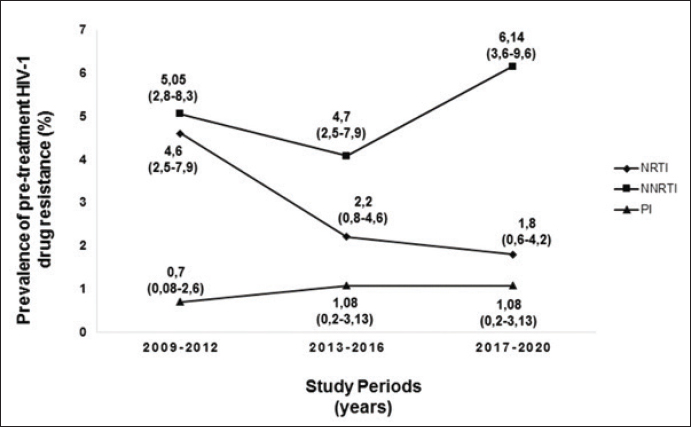
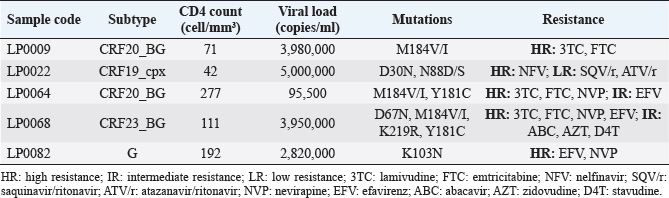
Figure 1 shows the trend in the behavior of pre-treatment resistance of HIV-1 to the main families of ARVs in the LPs studied in various periods between 2009 and 2020; observing a trend of increasing levels of resistance to NNRTIs over time (Fig. 1). 9.25% of the 52 LPs who presented HIV-1 variants with mutations associated with ARV resistance in the study died one year after the diagnosis of HIV infection. The CD4 cell count in the deceased LP ranged between 71 and 277 cells/mm3 (Table 3). DiscussionDespite the efforts of the international community to eliminate a group of barriers that prevent the achievement of the UNAIDS goals for the year 2030, there are several factors that persist in the HIV/AIDS pandemic (World Health Organization, 2022). Late presentation to health care and the presence of HIV variants resistant to ART are examples of these barriers. However, there are few studies in the world that describe the behavior of pre-treatment HIV-1 drug resistance in LPs and they are limited to developed countries (Huaman et al., 2011; Petersen et al., 2018; Miranda et al., 2022). In Latin America and the Caribbean, late presentation to health services of PLWH is one of the major problems and 30% of new HIV infections correspond to individuals with a CD4+ cell count of less than 200 cells/mm3 (PAHO, 2023). However, there are no reports on the behavior of HIV drug resistance in this group of individuals, so the present work is a pioneer in this type of research in the region. Table 2. Prevalence of pre-treatment HIV-1 drug resistance to antiretroviral families in Cuban PLWH classified as LPs (2009–2020).
Fig. 1. Behavior over time of pre-treatment HIV-1 drug resistance to different families of antiretrovirals in Cuban LPs studied between 2009 and 2020. NRTI: nuclesoside RT inhibitor; NNRTI: non-nucleoside transcriptase inhibitor; PI: protease inhibitor. Table 3. Characteristics of LPs with mutations associated with pre-treatment HIV-1 drug resistance who died one year after diagnosis of the infection.
The prevalence of pre-treatment HIV drug resistance is higher than that described by other studies that covered various periods of time (Huaman et al., 2011, Miranda et al., 2022), with a tendency to increase levels of resistance to the NNRTI. Since the introduction of ART in Cuba in 2001, nevirapine (NVP) or efavirenz (EFV) were among the NNRTIs used in the first therapeutic line until their gradual withdrawal based on the results of the national survey pre-treatment HIV-1 drug resistance in 2017 (Machado et al., 2019). These drugs favored the accumulation of mutations associated with resistance to this drug family (K103N, Y181C, and G190A) and the subsequent transmission of the variants with these changes to the population. Several pre-treatment HIV drug resistance studies have described an increase in resistance levels to NNRTIs in low- and middle-income countries (WHO, 2021). The trend of increasing pre-treatment HIV drug resistance to NNRTIs in Cuban LPs contrasts with the values described in a multicenter study carried out in Europe from 1981 to 2019, whose trend is towards a decrease (Miranda et al., 2022). 40% of LPs had CD4+ cell count values below 200 cells/mm3, indicating marked depression of their immune systems. In this group of people, possible reinfection with HIV-1 genetic variants different from the one causing the primary infection and that present mutations in their genome associated with resistance to ARVs, may be one of the causes of detection of pre-treatment HIV drug resistance. Pao et al. demonstrated that resistant variants can persist for up to three years in untreated individuals (Pao et al., 2004) and another research group proposed that they can be detected in patients with chronic infection and with a probable date of infection less than 10 years (Little et al., 2008, Machnowska et al., 2019). The presence of resistant viruses in sanctuary sites and the ability to integrate into the DNA of the host cell favors the persistence over time of HIV-1 variants resistant to ARVs (Chargin et al., 2015; Pasterak and Berkhout, 2023). Several studies have associated a higher prevalence of HIV-1 resistance mutations in subtype B than in non-B subtypes (Vercauteren et al., 2008, Li et al., 2015). In the present study, no association between the described viral variants and the presence of resistance mutations was found. However, a greater presence of mutations was detected in the URFs, which have increased their circulation in the Cuban seropositive population (Machado et al., 2017). The marked immunological deterioration of LPs leads to the appearance of opportunistic infections and diseases associated with AIDS (Antinori et al., 2011), and if we add the presence of mutations associated with the drugs used to control viral replication, the deterioration of health and subsequent death increase. The results described in the study are a clear example of the unfavorable outcome of late diagnosis of HIV infection. The results of pre-treatment HIV drug resistance in Cuban LP agree with the prevalence values detected in several studies carried out in Cuba in the untreated population (Machado et al., 2013, 2019, 2021; Pérez et al., 2013, 2020; Machado Zaldivar et al., 2022). The gradual withdrawal of EFV and NVP from first-line regimens in Cuba and the introduction of dolutegravir will contribute to reducing HIV pre-treatment resistance values in Cuba and reaching viral suppression values above 95%. However, active and timely research, the identification and characterization of social determinants in the epidemiological context of the country, will contribute to the search for new cases and the earlier diagnosis of the infection. ConclusionThe presence of ART-resistant variants in LP evidences the need to perform the HIV genotypic resistance test when diagnosing the infection, in conjunction with the detailed study of various social and epidemiological determinants, constituting an important tool in the management clinical status of these people, will contribute to meeting the goals set by UNAIDS for the year 2030. AcknowledgmentsThe authors would like to thank the doctors responsible for medical care in each of the municipalities selected for the survey, for their assistance in the collection of the samples and the clinical and demographic data of the patients participating in the survey. FundingThe survey was funded by the Global Fund to Fight AIDS, Tuberculosis, and Malaria (www.theglobalfund.org). The opinions referred to in the study are issued by the researchers involved in the study and do not represent the views or opinions of the Global Fund for the fight against AIDS, tuberculosis, and malaria. The Global Fund for the fight against AIDS, tuberculosis, and malaria is not involved in the approval of this manuscript. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. Conflict of interestThe authors do not declare conflicts of interest. Authors contributionsConceptualization: Liuber Y. Machado, Héctor M. Díaz, Liodelvio Martínez; Data curation: Liuber Y. Machado, Héctor M. Díaz, Neisy Valdés, Liodelvio Martínez, Karen Valdés; Formal analysis: Liuber Y. Machado, Madeline Blanco, Marta Dubed, Héctor M. Díaz, Liodelvio Martínez; Research: Liuber Y. Machado, Héctor M. Díaz, Madeline Blanco, Liodelvio Martínez, Enrique Noa, Laura S. López, Karen Valdés, Neisy Valdés; Methodology: Liuber Y. Machado, Héctor M. Díaz, Madeline Blanco, Liodelvio Martínez, María T. Pérez; Administration: Liuber Y. Machado; Supervision: Madeline Blanco, Marta Dubed, Otto Cruz, Mireida Rodríguez; Validation: Liuber Y. Machado, Héctor M. Díaz, María T. Pérez; Visualization: Liuber Y. Machado, Héctor M. Díaz, Liodelvio Martínez, Original draft: Liuber Y. Machado; Writing-review and editing: Liuber Y. Machado, Madeline Blanco, Marta Dubed, Héctor M. Díaz, María T. Pérez, Enrique Noa, Otto Cruz, Mireida Rodríguez. Data availabilityAll data are provided in the manuscript. ReferencesAlemán, Y., Vinken, L., Kourí, V., Pérez, L., Álvarez, A., Abrahantes, Y., Fonseca, C., Perez, J., Correa, C., Soto, Y. and Schrooten, Y. 2015. Performance of an in-house human immunodeficiency virus type 1 genotyping system for assessment of drug resistance in Cuba. PLoS One 10(2), e0117176. Antinori, A., Coenen, T., Costagiola, D., Dedes, N., Ellefson, M., Gatell, J., Girardi, E., Johnson, M., Kirk, O., Lundgren, J. and Mocroft, A. 2011. Late presentation of HIV infection: a consensus definition. HIV Med. 12, 61–64. Bennett, D.E., Camacho, R.J., Otelea, D., Kuritzkes, D.R., Fleury, H., Kiuchi, M., Heneine, W., Kantor, R., Jordan, M.R., Schapiro, J.M. and Vandamme, A.M. 2009. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One. 4(3), e4724. Chargin, A., Fangfang, Y., Sang, M., Srividyabhuvaneswari, S., Knutsan, G., Patterson, B.K. 2015. Identification and characterization of HIV-1 latent viral reservoirs in peripheral blood. J. Clin. Microb. 53(1), 60–66. Díaz, H.M., Pérez, M.T., Lubián, A.L., Nibot, C., Cruz, O., Silva, E., Rolo, F. and Izquierdo, M. 2011. HIV detection in Cuba: role and results of the national laboratory network. Med Rev. 13(2), 9–13. Guaraldi, G., Zona, S., Menozzi, M., Brothers, T.D., Carli, F., Stentarelli, C., Dolci, G., Santoro, A., Beghetto, B., Menozzi, M., Mussini, C. and Falutz, J. 2017. Late presentation increases risk and costs of non-infectious comorbidities in people with HIV: an Italian cost impact study. AIDS Res, Ther. 14(8), 1–7. Günthard, H.F., Aberg, J.A., Eron, J.J., Hoy, J.F., Telenti, A., Benson, C.A., Burger, D.M., Cahn, P., Gallant, J.E., Glesby, M.J. and Reiss, P. 2014. Antiretroviral treatment of adults HIV infection. Recommedations of the International Antiviral Society USA Panel. JAMA. 312(4), 410–425. Huaman, M.A., Aguilar, J., Baxa, D., Golembieski, A., Brar, I. and Markowitz, N. 2011. Late presentation and transmitted drug resistance mutations in new HIV-1 diagnoses in Detroit. Int. J. Infect. Dis. 15, e764–8. Li, Y., Gu, L., Han, Y., Xie, J., Wang, H., Lv, W., Song, X., Li, Y., Iwamoto, A., Ishida, T. and Li, T. 2015. HIV-1 subtype B/B’ and baseline drug resistance mutation are associated with virologic failure: a multicenter cohort study in China. J. Acquir. Immune DeficSyndr. 68(3), 289–97. Little, S., Frost, S., Wong, J.K., Smith, S., Pond, S.K., Ignacio, C., Parkin, N.T., Petropoulos, C.J. and Richman, D.D., 2008. Persistence of transmitted drug resistance among subjects with primary human immunodeficiency virus infection. J. Virol. 82(11), 5510–5518. Machado, L.Y., Blanco, M., López, L.S., Díaz, H.M., Dubed, M., Valdés, N., Noa, E., Martínez, L., Perez, M.T., Romay, D.M. and Rivero, C.B. 2019. National survey of pre-treatment HIV drug resistance in Cuban patients. PLOS One 14(9), e0221879. Machado, L.Y., Dubed, M., Díaz, H.M., Ruiz, N., Romay, D., Valdés, N., Blanco, M. and Silva, E. 2013. Transmitted HIV type 1 drug resistance in newly diagnosed Cuban patients. AIDS Res. Hum. Retroviruses. 29(2), 411–414. Machado, L.Y., Pintos, Y., Diaz, H.M., Perez, L., Blanco, M., Kouri, V., Aleman, Y., Martinez, L., Dubeda, M., Aragones, C., Ruiz, N., Silva, E., Soto, Y., Valdes, N., Banos, Y., Caturla, Y., Romay, D., Perez, J., Nibot, C., Rocha, N., Rodriguez, R., Sanchez, M.L. and Trinquete, A.A. 2017. Increase of recombinant forms CRF20, 23, 24_BG and several URF of HIV-1 among newly diagnosed Cuban patients: 2013–2014. ARC J AIDS. 2(1):24–31. Machado Zaldivar, L.Y., Blanco de Armas, M., Dubed Echevarría, M., Díaz Torres, H.M., Romay-Franchi, D., Valdés–de Calzadilla, N., Noa Romero, E., López Rizo, L.S., Pérez Guevara, M.T., Suárez Batista, A. and Rodríguez Acosta, M. 2022. Resistencia pretratamiento del virus de inmunodeficiencia humana tipo 1 a fármacos antirretrovirales en Cuba: 2009–2017. Acad Cienc Cuba 12(3), e1154. Machado-Zaldivar, L.Y., Blanco-de Armas, M., Dubed-Echevarría, M., Díaz-Torres, H.M., López Rizo, L.S., Pérez, Guevara, M.T., Lantero-Abreu, M.I. and Rodríguez-Acosta, M. 2021. Pre-treatment HIV drug resistance surveillance as a tool for monitoring and control of HIV/AIDS epidemic in Cuba. MEDICC Rev.; 23(2), 64–68. Machnowska, P., Meixenberger, K., Schmidt, D., Jessen, H., Hillenbrand, H., Gunsenheimer-Bartmeyer, B., Hamouda, O., Kücherer, C., Bannert, N. and German HIV-1 Seroconverter Study Group 2019. Prevalence and persistence of transmitted drug resistance mutations in the German HIV-1 Seroconverter Study Cohort. PLoS One 14(1), e0209605. Miranda, M.N.S., Pingarilho, M., Pimentel, V., Martins, M.d.R.O., Kaiser, R., Seguin-Devaux, C., Paredes, R., Zazzi, M., Incardona, F. and Abecasis, A.B. 2022. Trends of transmitted and acquired drug resistance in Europe From 1981 to 2019: a comparison between the populations of late presenters and non-late presenters. Front. Microbiol. 13, 846943. doi:10.3389/fmicb.2022.846943. PAHO. 2023. Washington, DC: Pan American Health Organization. Available via https://paho.org/tema/vih-sida (Accessed 26 January 2024). Pao, D., Andrady, U., Clark, J., Dean, G., Darke, S., Fisher, M., Green, T., Kumar, S., Murphy, M., Tang, A. and Taylor, S. 2004. Long term persistence of primary genotypic resistance after HIV-1 seroconversion. J. Acquir. Immune DeficSyndr. 37:1570–1573. Pasterak, A.O. and Berkhout B. 2023. HIV persistence: silence or resistance? Current Opin. Virol. 59, 101301. Pérez L, Kouri V, Aleman Y, Abrahantes Y, Correa C, Aragonés C, et al. 2013. Antiretroviral drug resistance in HIV-1 therapy-naive patients in Cuba. Infect Genet Evol. 16C: 144–150. Pérez, L., Machado, L.Y., Pintos, Y., Díaz, H.M., Kourí, V., Aragonés, C., Correa, C., Alemán, Y., Silva, E., Blanco de Armas, M., Pérez, L.J., Muné, M., Dubed, M., Soto, Y., Ruiz, N., Limia, C.M., Nibot, C., Valdés, N., Ortega, L.M., Romay, D., Campos, Y., Rivero, C.B. and Campos, J. 2020. Antiretroviral drug resistance transmitted in HIV-1 newly diagnosed Cuban patients. April 2013–April 2014. Clin. Res HIV/AIDS 7(1), 250. Petersen, A., Cowan, S.A., Nielsen, J., Fischer, T.K., Fonager, J. 2018. Characterisation of HIV-1 transmission clusters and drug-resistant mutations in Denmark, 2004 to 2016. Euro Surveill 23(44), pii=1700633. Quarleri, J.F., Rubio, A., Carobene, M., Turk, G., Vignoles, M., Harrigan, R.P., Montaner, J.S., Salomón, H. and Gómez-Carrillo, M. 2004. HIV type 1 BF recombinant strains exhibit different pol gene mosaic patterns: descriptive analysis from 284 patients under treatment failure. AIDS Res. Hum. Retroviruses. 20(10), 1100–1117. Tang, M.W. and Shafer, R.W. 2012. HIV antiretroviral resistance. Scientific principles and clinical applications. Drug. 72(9), e1–e25. Vercauteren, J., Derdelinckx, I., Sasse, A., Boguert, M., Ceunen, H., De Roo, A., De Wit, S., Deforche, K., Echahidi, F., Fransen, K. and Goffard, J.C. 2008. Prevalence an epidemiology of HIV type 1 drug resistance among newly diagnosed therapy-naïve patients in Belgium from 2003 to 2006. AIDS Res. Hum. Retroviruses. 24(3), 355–362. World Health Organization. 2021. HIV drug resistance report 2021. Geneva, Switzerland: World Health Organization. World Health Organization. 2022. Global health sector strategies on respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022–2030. Geneva, Switzerland: World Health Organization. World Health Organization. 2023. Providing care to people with advanced HIV disease who are seriously ill: policy brief. Geneva, Switzerland: World Health Organization. World Health Organization. 2016. Global health sector strategy on HIV. 2016–2021. Towards ending aids. Geneva, Switzerland: World Health Organization. Available via https://apps.who.int/iris/bitstream/handle/10665/246178 World Health Organization. 2017. Global action plan on HIV drug resistance 2017–2021. Geneva, Switzerland: World Health Organization, pp: 1–37. | ||
| How to Cite this Article |
| Pubmed Style Machado LY, Díaz HM, Martínez L, Blanco M, Dubed M, Valdés N, Valdés K, López LS, Noa E, Pérez MT, Cruz O, Rodríguez M. Pre-treatment HIV-1 drug resistance in Cuban patients with late presentation to health care: 2009-2020.. J Microbiol Infect Dis. 2024; 14(2): 60-66. doi:10.5455/JMID.2024.v14.i2.4 Web Style Machado LY, Díaz HM, Martínez L, Blanco M, Dubed M, Valdés N, Valdés K, López LS, Noa E, Pérez MT, Cruz O, Rodríguez M. Pre-treatment HIV-1 drug resistance in Cuban patients with late presentation to health care: 2009-2020.. https://www.jmidonline.org/?mno=194137 [Access: July 14, 2025]. doi:10.5455/JMID.2024.v14.i2.4 AMA (American Medical Association) Style Machado LY, Díaz HM, Martínez L, Blanco M, Dubed M, Valdés N, Valdés K, López LS, Noa E, Pérez MT, Cruz O, Rodríguez M. Pre-treatment HIV-1 drug resistance in Cuban patients with late presentation to health care: 2009-2020.. J Microbiol Infect Dis. 2024; 14(2): 60-66. doi:10.5455/JMID.2024.v14.i2.4 Vancouver/ICMJE Style Machado LY, Díaz HM, Martínez L, Blanco M, Dubed M, Valdés N, Valdés K, López LS, Noa E, Pérez MT, Cruz O, Rodríguez M. Pre-treatment HIV-1 drug resistance in Cuban patients with late presentation to health care: 2009-2020.. J Microbiol Infect Dis. (2024), [cited July 14, 2025]; 14(2): 60-66. doi:10.5455/JMID.2024.v14.i2.4 Harvard Style Machado, L. Y., Díaz, . H. M., Martínez, . L., Blanco, . M., Dubed, . M., Valdés, . N., Valdés, . K., López, . L. S., Noa, . E., Pérez, . M. T., Cruz, . O. & Rodríguez, . M. (2024) Pre-treatment HIV-1 drug resistance in Cuban patients with late presentation to health care: 2009-2020.. J Microbiol Infect Dis, 14 (2), 60-66. doi:10.5455/JMID.2024.v14.i2.4 Turabian Style Machado, Liuber Y, Héctor M Díaz, Liodelvio Martínez, Madeline Blanco, Marta Dubed, Neisy Valdés, Karen Valdés, Laura S López, Enrique Noa, María T Pérez, Otto Cruz, and Mireida Rodríguez. 2024. Pre-treatment HIV-1 drug resistance in Cuban patients with late presentation to health care: 2009-2020.. Journal of Microbiology and Infectious Diseases, 14 (2), 60-66. doi:10.5455/JMID.2024.v14.i2.4 Chicago Style Machado, Liuber Y, Héctor M Díaz, Liodelvio Martínez, Madeline Blanco, Marta Dubed, Neisy Valdés, Karen Valdés, Laura S López, Enrique Noa, María T Pérez, Otto Cruz, and Mireida Rodríguez. "Pre-treatment HIV-1 drug resistance in Cuban patients with late presentation to health care: 2009-2020.." Journal of Microbiology and Infectious Diseases 14 (2024), 60-66. doi:10.5455/JMID.2024.v14.i2.4 MLA (The Modern Language Association) Style Machado, Liuber Y, Héctor M Díaz, Liodelvio Martínez, Madeline Blanco, Marta Dubed, Neisy Valdés, Karen Valdés, Laura S López, Enrique Noa, María T Pérez, Otto Cruz, and Mireida Rodríguez. "Pre-treatment HIV-1 drug resistance in Cuban patients with late presentation to health care: 2009-2020.." Journal of Microbiology and Infectious Diseases 14.2 (2024), 60-66. Print. doi:10.5455/JMID.2024.v14.i2.4 APA (American Psychological Association) Style Machado, L. Y., Díaz, . H. M., Martínez, . L., Blanco, . M., Dubed, . M., Valdés, . N., Valdés, . K., López, . L. S., Noa, . E., Pérez, . M. T., Cruz, . O. & Rodríguez, . M. (2024) Pre-treatment HIV-1 drug resistance in Cuban patients with late presentation to health care: 2009-2020.. Journal of Microbiology and Infectious Diseases, 14 (2), 60-66. doi:10.5455/JMID.2024.v14.i2.4 |