| Research Article | ||
J. Microbiol. Infect. Dis., (2023), Vol. 13(2): 53–58 Original Research Prevalence of carbapenemase-producing organisms among patients admitted in intensive care unit in a tertiary hospital in Benin city, NigeriaEphraim E. Ibadin1,2*, Helen O. Ogefere2, Richard Omoregie1 and Jeremiah A. Igunma31Medical Microbiology Unit, Medical Laboratory Services, University of Benin Teaching Hospital, Benin City, Nigeria 2Department of Medical Laboratory Science, School of Basic Medical Sciences, University of Benin, Benin City, Nigeria 3Medical Microbiology Department, University of Benin Teaching Hospital, Benin City, Nigeria *Corresponding Author: Ephraim E. Ibadin. Medical Microbiology Unit, Medical Laboratory Services, University of Benin Teaching Hospital, Benin City, Nigeria. Email: ibadinsmailbox [at] yahoo.com Submitted: 13/12/2022 Accepted: 04/06/2023 Published: 30/06/2023 © 2023 Journal of Microbiology and Infectious Diseases
AbstractAim: The study aimed to determine the prevalence of carbapenemase-producing organisms (CPOs) causing clinical infections among intensive care unit (ICU) patients at the University of Benin Teaching Hospital, Benin City, Nigeria. Methods: Gram negative bacterial isolates recovered from clinical specimens of patients admitted at the ICU of the hospital during the study period were identified using Microbact 20E and antimicrobial susceptibility tests carried out. Carbapenem resistant isolates were thereafter screened phenotypically for carbapenemase production, CPOs were subsequently screened using PCR for the following genes; NDM, VIM, KPC and OXA-48-like. Results: A total of 64 clinical specimens were received during the study period. Of this number, 26 (40.6%) were culture positive for Enterobacterales (21.9%) and non-fermenters (18.8%). Amikacin showed the best susceptibility profile with 81.5% overall activity against all isolates, the carbapenems showed moderate activity with 66.7% while the third generation cephalosporins were poorly active (37%) against all bacterial isolates. Carbapenemase activity was observed in 9 isolates (14.1%), one isolate of Enterobacter cloacae was VIM positive while 62.5% and 25% of CP-Pseudomonas aeruginosa were NDM and VIM positive respectively. Conclusion: Carbapenemase-producing-P. aeruginosa was the leading cause of infections among ICU patients in Benin City, Nigeria. There is therefore need for surveillance, IPC measures and adherence of antimicrobial stewardship guidelines at institutional and national levels. Keywords: Carbapenemase, Resistance, Clinical infection, Intensive care unit, Bacterial isolate. IntroductionThe intensive care unit (ICU) is specially designed and equipped to cater to individuals who have suffered from serious injury, illness, or have a medical complication in their respiratory, renal, cardiac or nervous system following surgical intervention (Al Fadhli et al., 2020). Patients in ICUs have been increasingly associated with healthcare associated infections (HAI) due to multidrug resistant (MDR) pathogens (Alebel et al., 2021). Predisposing factors for their risk of HAIs include their weakened immune status, the use of invasive medical devices as well as administration of multiple antimicrobial drugs (Al Fadhli et al., 2020; Alebel et al., 2021). These are thus contributory factors to the morbidity and mortality increasingly observed among ICU patients (Alebel et al., 2021). Globally, infections with Gram-negative bacilli are 5–10 times higher among patients in the ICU than those in the general wards (Jean et al., 2022). Indeed, among the ESKAPE pathogens, Klebsiella pneumoniae and Pseudomonas aeruginosa of different sequence types have been increasingly associated with MDR infections and local outbreaks in ICU wards (Aminu et al., 2021; Jean et al., 2022). This may be worsened in environments where infection prevention and control (IPC) practices are not deeply entrenched as has been observed in most low- and middle-income countries (Parajuli et al., 2017; Jean et al., 2022). Carbapenemase-producing organisms (CPO) are a global concern due to the fact that they are resistant to several classes of antimicrobials (MDR) including carbapenems- the drugs of last resort in several settings (Al Fadhli et al., 2020; Jean et al., 2022). Their isolation from clinical specimens of patients in ICU further worsens their treatment outcomes, leading to increased length of stay in the wards, cost, morbidity, and mortality (Jean et al., 2022). The global prevalence of CPOs causing infections in ICU has been shown to vary according to region, in relation to organism, sequence type, and carbapenemase type (Al Fadhli et al., 2020; Baban et al., 2020). Few studies have been done on this subject in Nigeria. In Kano, North-western Nigeria, 9.2% of Enterobacteriaceae recovered from clinical specimens of patients in ICU were CPOs (Aminu et al., 2021). We had previously explored the prevalence of CPO causing clinical infections in Benin City, Southern Nigeria, and highlighted the high prevalence of NDM gene being associated with P. aeruginosa (Ibadin et al., 2022). Owing to the paucity of data in Southern Nigeria, this study sought to determine the prevalence of CPOs causing clinical infections among ICU patients at the University of Benin Teaching Hospital (UBTH), Benin City, Nigeria. Materials and MethodsStudy area and designBenin City is an urban area and capital city of Edo state, southern Nigeria. The study was cross-sectional and was conducted at the UBTH, Benin City, Edo State, Nigeria. The hospital is comprised of 20 wards and 850 beds, being a tertiary health institution serving the specialist health needs of the state’s teeming population and six neighboring states. Sample processing/ bacterial isolatesClinical specimens of patients in the ICU showing signs and symptoms of infections were collected aseptically into appropriate containers and sent to the Medical Microbiology Laboratory, UBTH. The specimens included urine, swabs, blood, urinary catheter tip, tracheal aspirate, cerebrospinal fluid, and sputum. These were processed within 2 hours of receipt of the specimens according to standard procedures. Emergent and significant cultures that were Gram Negative rods were identified using Microbact 20E. Antimicrobial susceptibility testAntimicrobial susceptibility testing of all isolates against different antibiotics was determined by the disc diffusion method on Mueller-Hinton agar plate according to the Clinical Laboratory Standards Institute (2020). After seeding the test bacillus on the plate, the following antibiotics were placed aseptically: Meropenem (10 µg), Imipenem (10 µg), Ceftazidime (30 µg), Ceftriaxone (30 µg), Cefuroxime (30 µg), Levofloxacin (5 µg), Amoxicillin-clavulanate (30 µg) and Amikacin (30 µg). These were then incubated at 37°C for 16 to 24 hours, and the diameter of the zones of inhibition was determined by the breakpoints of antimicrobial discs measured and interpreted according to the CLSI guidelines (Clinical and Laboratory Standards Institute (CLSI), 2020). Simplified carbapenemase inactivation method (sCIM)Bacterial isolates that were resistant to imipenem and/or meropenem were screened for carbapenemase. A modification of the sCIM described by Jing et al. (2018) was used. Briefly, a 0.5 McFarland standard suspension of the indicator strain ( Escherichia coli ATCC 25922) was swabbed in three directions on a Mueller Hinton agar (MHA) plate and allowed to dry for 3–10 minutes. One to three colonies of the test bacillus which had grown on blood agar was thereafter smeared on one side of 10µg imipenem discs (from Oxoid, UK). The side of the antibiotic discs smeared with the test organism was immediately put on the already seeded Mueller Hinton plate and incubated at 35°C for 16–18 hours. Another imipenem disk (not smeared with organism) was placed on the MHA plate to serve as a control. For the interpretation of the test, discs with a zone diameter ≤22 mm infers that the isolate is carbapenemase-producing (positive); a zone of inhibition ≥26 mm infers the absence of carbapenemase activity (negative); a zone of inhibition of 23–25 mm infers a carbapenemase indeterminate isolate (9). Bacterial isolates that were carbapenemase positive or intermediate were included for Polymerase chain reaction (PCR) detection of carbapenemase genes. Identification of carbapenemase genesPCR was used to identify genes encoding various known carbapenemases. DNA extracts were obtained by boiling method and multiplex PCR amplification for the simultaneous detection of blaKPC, blaNDM, blaOXA-48-like, and blaVIM, β-lactamase genes was carried out on a Veriti 96-well thermal cycler instrument (Applied Biosystems at Life Technologies, Foster City, CA) with the AmpliTaq Gold PCR master mix (Applied Biosystems at Life Technologies, Hammonton, NJ) as previously described (Doyle et al., 2012). The primers were used at concentrations of 0.3 µM each for blaKPC, and blaVIM, 0.4 µM for blaNDM, and 0.5 µM for blaOXA-48-like. The primer sequences and amplicon sizes are shown in (Table 1). The PCR products were analyzed by electrophoresis with 1.5% agarose gels in 0.5× Tris-borate-EDTA buffer. The gels were stained with SYBR Safe DNA gel stain (Invitrogen, Portland, Oregon), and the PCR products were visualized with UV light (Fig. 1). The molecular tests were carried out at the clinical section of Microbiology, Calgary laboratory services, Alberta, Canada. Table 1. Primers used for amplification of carbapenemase genes.
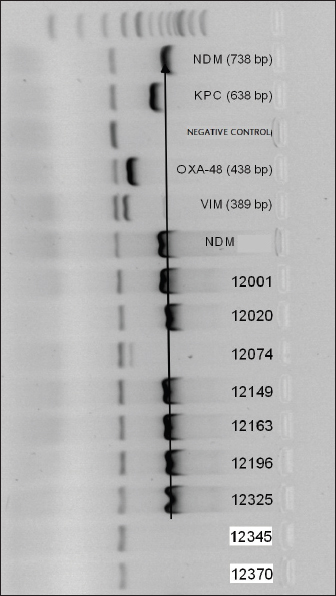
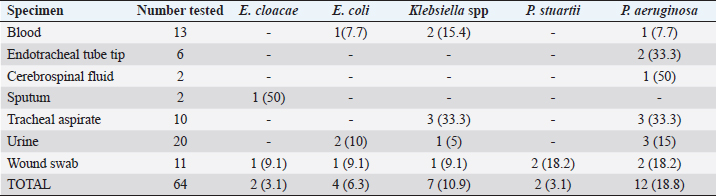
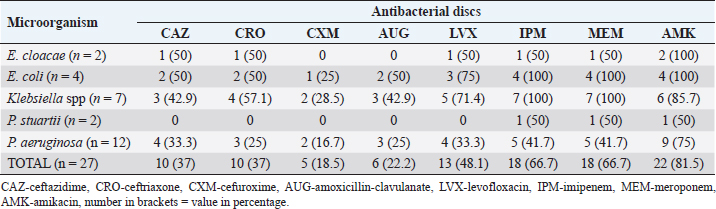
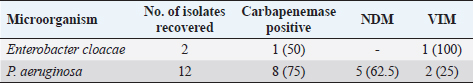
Fig. 1. Electrophoresis gel of multiplex PCR run for carbapenemase producing organisms from ICU, UBTH. The first five isolates are the positive and negative control isolates NDM, KPC, negative control (E. coli ATCC 2), OXA-48-like, and VIM genes. Isolates’ 12001, 12020, 12149, 12163, 12196 and 12325 are positive for NDM gene, while isolate 12074 is positive for VIM gene. ResultsA total of 64 clinical specimens were received during the study period comprising body fluid specimens (blood, cerebrospinal fluid, and tracheal aspirate), catheter tips, urine, and wound swabs. Of this number, 26 (40.6%) were culture positive for Enterobacterales (21.9%) and non-fermenters (18.8%). Pseudomonas aeruginosa (18.8%) was the most recovered Gram-negative bacillus while Providentia stuartii (1.6%) showed the least prevalence (Table 2). Amikacin showed the best susceptibility profile with 100% activity against E. cloacae and E. coli and 81.5% overall activity against all Gram-negative bacterial isolates. Cefuroxime (18.5%) and amoxicillin-clavulanate (22.2%) were the least active antibacterial drugs tested. The carbapenems (imipenem and meropenem) showed moderate activity with 66.7% while the third generation cephalosporins (ceftazidime and ceftriaxone) were poorly active (37%) against all bacterial isolates (Table 3). Using the sCIM carbapenemase detection method, 9 (14.1%) bacterial isolates showed carbapenemase activity. These isolates were then screened using PCR method. However, 8 bacterial isolates were PCR positive (Fig. 1). One isolate of E. cloacae was VIM positive while 62.5% and 25% of CP-P. aeruginosa were NDM and VIM positive respectively (Table 4). Table 2. Distribution of bacterial isolates recovered from ICU in relation to clinical specimens.
Table 3. Antimicrobial susceptibility profile of Gram-negative bacilli recovered from clinical specimens of patients from intensive care unit (ICU), UBTH.
Table 4. Distribution of carbapenemase genes among Gram negative bacterial isolates recovered from patients admitted in ICU, UBTH.
DiscussionCPOs are problematic and of global concern as these organisms could be MDR, pan-drug resistant or extensively drug resistant leading to summarily limited therapeutic options for patients (Ibadin et al., 2022; Jing et al., 2018). As treatment outcomes may be comparatively poorer in low- and middle-income countries due to limited access to quality healthcare and poor adherence to IPC practices, this study evaluated the prevalence of these organisms among critically ill intensive care patients in a tertiary hospital, Southern Nigeria. The most frequently isolated organism causing infections among ICU patients in this study was P. aeruginosa (18.8%). This finding is in contrast to a study carried out in Kano, Northern Nigeria which showed E. coli (44.4%) as having the highest prevalence (Aminu et al., 2021). In that study however, emphasis was placed on the Enterobacterales. The finding is similar to a study in Indonesia in which P. aeruginosa was the leading etiologic agent (26.5%) (Radji et al., 2011), but differs from three Asian studies; In India, the leading etiologic agent was E. coli (18.6%) while in Saudi Arabia and Nepal, Acinetobacter spp were reported to be the most prevalent among ICU patients (Parajuli et al., 2017; Ibrahim, 2018; Savanur and Gururaj, 2019). In Latin America and the Caribbean, Gram-negative pathogens, predominantly Acinetobacter baumannii, Klebsiella pneumoniae, P. aeruginosa, and E. coli, account for over 50% of ICU infections (Luna et al., 2014). The etiologic agents of infections in ICU may therefore vary across geographical regions, this may explain our finding. The most active antibacterial drug in this study was amikacin (81.5%). This finding is similar to a study in Indonesia where 84.4% of Gram-negative bacterial isolates were susceptible to the drug (Radji et al., 2011). The finding however differs from a study on similar subjects in India, where moderate activity was observed for the drug (Savanur and Gururaj, 2019). Amikacin is a drug of last resort in our setting (Institutional antibiotic policy). Our finding, therefore, calls for vigilance as there is brewing resistance to the antibacterial drug. Similarly, the third generation cephalosporins showed poor activity against bacterial isolates. The finding is similar to a study in Kano, Nigeria where high resistance rates were observed to this class of antibacterials (Aminu et al., 2021). Our observation was also in agreement with studies in Saudi Arabia, India, and Indonesia where high resistance was observed to these drugs (Radji et al., 2011; Ibrahim et al., 2018). Abuse of the third generation cephalosporins has been highlighted in Nigeria and high prevalence of extended spectrum beta-lactamase-producing isolates has been reported in the study area in recent times, being predominantly CTX-M-15 variants (Radji et al., 2011; Jesumirhewe et al., 2020). Worryingly, the carbapenems- a beta-lactam of last resort showed moderate activity in this study. Reduced susceptibility to carbapenems have been documented in ICUs (Baban, 2020; Aminu et al., 2021). Carbapenem resistance could be intrinsic (impermeability of outer cell membrane, efflux pumps) or enzyme-mediated (production of carbapenemase enzyme). Several genotypes that have been associated with carbapenemase production include NDM, VIM, OXA48, OXA-181, IMP, KPC, GES, etc. Their epidemiology is influenced by international travel and selective pressure and therefore varies across regions (Logan and Weinstein, 2017; Hansen, 2021). The prevalence of CPO in this study was 14.1% and two carbapenemase genes were detected (NDM and VIM) with NDM being predominant. In Kano, a prevalence of 10.5% was observed with VIM gene predominating (Aminu et al., 2021). Recent studies on clinical isolates from Nigeria have shown a rise in the metallo-beta-lactamase producing Gram-negative bacteria with the NDM type being the most common (Mohammed et al., 2015; Jesumirhewe et al., 2017; Ibadin et al., 2022). Strikingly, 75% of P. aeruginosa recovered were CPOs with NDM and VIM genes being predominant. Similar findings were observed in Iraq and Egypt, where the dominant gene type among P. aeruginosa isolates from ICU patients were of the VIM and NDM types (ElDomany et al., 2017; Baban, 2020). Pseudomonas aeruginosa seems adapted for the hospital environment as most strains are typically resistant to antiseptics, have intrinsic resistance to several antibiotics (due to the low permeability of its outer membrane and constitutive expression of efflux pumps), and the capability for genetic transfer of antibacterial resistance (Baban, 2020). Some studies have shown that as time from patient’s admission increases, there is a linear increase in the likelihood of isolation of P. aeruginosa (Daneman et al., 2012). Recent studies have also shown that the risk of colonization with CP-P. aeruginosa isolates harboring VIM-type metallo-beta-lactamase increases with the length of hospital stay (especially above 30-day durations) (Daneman et al., 2012; Neidhöfer et al., 2021). Although this study did not highlight the duration of stay of study participants, as with most ICUs, most participants had prolonged hospital stay due to morbidity. This may therefore explain our findings and brings to the fore the need for targeted IPC measures. ConclusionThe prevalence of CPO in this study was 14.1%. Carbapenemase-producing-P. aeruginosa was the leading cause of infections among ICU patients in Benin City, Nigeria, and NDM and VIM were the dominant genotypes. Therefore, surveillance, IPC measures, and adherence to antimicrobial stewardship guidelines at institutional and national levels are needed. AcknowledgmentsThe authors are grateful to Prof Pitout J and Dr Peirano Gisele for the logistic support and critical notes during the course of this research. ReferencesAl Fadhli, A.H., Jamal, W.Y. and Rotimi, V.O. 2020. Prevalence of carbapenem-resistant Enterobacteriaceae and emergence of high rectal colonization rates of blaOXA-181-positive isolates in patients admitted to two major hospital intensive care units in Kuwait. PLoS One. 15(11), e0241971; https://doi.org/10.1371/journal.pone.0241971 Alebel, M., Mekonnen F. and Mulu, W. 2021. Extended-Spectrum β-Lactamase and carbapenemase-producing Gram-negative bacilli infections among patients in intensive care units of Felegehiwot Referral Hospital: a prospective cross-sectional study. Infect. Drug Resist. 14, 391–405. Aminu, A., Daneji, M.I., Yusuf, M.A., Jalo, R.I., Tsiga-Ahmed, F.I., Yahaya, M., Adamu A.A., Yaqub, Y., Dayyab, F.M., Edwin, C.P. and Garba, S2021. Carbapenemresistant Enterobacteriaceae infections among patients admitted to intensive care units in Kano, Nigeria. Sahel Med. J. 24, 1–9. Baban ST. 2020. Molecular detection of carbapenemaseproducing Pseudomonas aeruginosa isolated from intensive care units of surgical specialty hospital in Erbil city. Med. J. Babylon. 17:185–193. Clinical and Laboratory Standards Institute (CLSI). 2020. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100. 30th ed. Wayne, PA: Clinical and Laboratory Standards Institute Doyle, D., Peirano, G., Lascols, C., Lloyd, T., Church, D.L. and Pitout, J.D.D. 2012. Laboratory detection of Enterobacteriaceae that produce carbapenemases. J. Clin. Microb. 50(12), 3877. ElDomany, R.A., Emara, M., ElMagd, M.A., Moustafa, WH. and Abdeltwab, N.M. 2017. Emergence of imipenemresistant Pseudomonas aeruginosa clinical isolates from Egypt coharboring VIM and IMP carbapenemases. Microb. Drug Resist. 23, 682–686. Daneman, N., Elligsen, M., Walker, S.A. and Simor, A. 2012. Duration of hospital admission and the need for empirical antipseudomonal therapy. J. Clin. Microbiol. 50(8), 2695–2701. Ibadin E.E., Ogefere, H.O, Peirano, G. and Pitout J. 2022. High prevalence of NDM genes among carbapenemase-producing clinical Gram negative bacilli in Benin City, Nigeria: Pseudomonas aeruginosa-a leading culprit. N. Z. J. Med. Lab. Sci. 76(3), 90–92. Ibrahim, M.E. 2018. High antimicrobial resistant rates among Gram-negative pathogens in intensive care units. A retrospective study at a tertiary care hospital in Southwest Saudi Arabia. Saud. Med. J. 39(10), 1035–1043; doi: 10.15537/smj.2018.10.22944 Ibadin, E.E., Omoregie, R., Igbarumah, O.I., Anogie, N.A. and Ogefere, H.O. Prevalence of extended spectrum β-lactamase, AmpC β-lactamase and metallo-β-lactamase among gram negative bacilli from clinical specimens in a tertiary hospital in Benin City, Nigeria. Int. J. Enter Path. 5(3), 85–91. Hansen G. 2021. Continuous evolution: perspective on the epidemiology of carbapenemase resistance among Enterobacterales and other Gram-negative bacteria. Infect. Dis. Ther. 10, 75–92; https://doi.org/10.1007/s40121-020-00395-2 Jesumirhewe, C., Springer, B., Lepuschitz, S., Allerberger, F. and Ruppitsch, W. 2017. Carbapenemase-producing Enterobacteriaceae-isolates from Edo State, Nigeria. Antimicrob. Ag. Chemother. 61, e00255–e00217; https://doi.org/10.1128/AAC.00255-17. Jesumirhewe, C., Springer, B., Allerberger, F. and Ruppitsch, W. 2020. Whole genome sequencing of extended-spectrum β-lactamase genes in Enterobacteriaceae isolates from Nigeria. PLoS One. 15(4), e0231146; https://doi.org/10.1371/journal.pone.0231146 Jean, S-S., Harnod, D. and Hsueh, P-R. 2022. Global threat of carbapenem-resistant Gram-negative bacteria. Front. Cell. Infect. Microbiol. 12, 823684; doi: 10.3389/fcimb.2022.823684 Jing, X., Zhou, H., Min, X., Zhang X., Yang, Q., Du, S., Li, Y., Yu, F., Jia, M., Zhan, Y. and Zeng Y.. 2018. The simplified carbapenem inactivation method (sCIM) for simple and accurate detection of carbapenemase-producing Gram-negative Bacilli. Front. Microbiol. 9, 2391; doi: 10.3389/fmicb.2018.02391 Luna, C.M., Rodriguez-Noriega, E., Bavestrello, L. and Guzmán-Blanco, M. 2014. Gram-negative infections in adult intensive care units of Latin America and the Caribbean. Crit. Care Res. Pract. 2014, 480463. Logan, L.K. and Weinstein, R.A. 2017. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J. Infect. Dis. 215(S1):S28–S36. Mohammed, Y., Zailani, SB. and Onipede, O.A. 2015. Characterization of KPC, NDM and VIM Type carbapenem resistance Enterobacteriaceae from North Eastern, Nigeria. J. Biosci. Med. 3, 100–107 Neidhöfer, C., Buechler, C., Neidhöfer, G., Bierbaum, G., Hannet, I., Hoerauf, A. and Parčina, M. 2021. Global distribution patterns of carbapenemase-encoding bacteria in a new light: clues on a role for ethnicity. Front. Cell. Infect. Microbiol. 11, 659753; doi: 10.3389/fcimb.2021.659753 Parajuli, N.P., Acharya, S.P., Mishra, S.K. Parajuli, K., Rijal, B.P. and Pokhrel, B.M. 2017. High burden of antimicrobial resistance among gram negative bacteria causing healthcare associated infections in a critical care unit of Nepal. Antimicrob. Resist. Infect. Control. 6 (67), 1–9; https://doi.org/10.1186/s13756-017-0222-z Radji, M., Fauziah, S. and Aribinuko, N. 2011. Antibiotic sensitivity pattern of bacterial pathogens in the intensive care unit of Fatmawati Hospital, Indonesia. Asian Pac. J. Trop. Biomed. (1), 39–42. Savanur SS and Gururaj H. 2019. Study of antibiotic sensitivity and resistance pattern of bacterial isolates in intensive care unit setup of a tertiary care hospital. Indian J. Crit. Care Med. 23(12), 547–555. | ||
| How to Cite this Article |
| Pubmed Style Ibadin EE, Ogefere HO, Omoregie R, Igunma J. Prevalence of carbapenemase-producing organisms among patients admitted to intensive care unit in a tertiary hospital in Benin city, Nigeria. J Microbiol Infect Dis. 2023; 13(2): 53-58. doi:10.5455/JMID.2023.v13.i2.2 Web Style Ibadin EE, Ogefere HO, Omoregie R, Igunma J. Prevalence of carbapenemase-producing organisms among patients admitted to intensive care unit in a tertiary hospital in Benin city, Nigeria. https://www.jmidonline.org/?mno=302657411 [Access: January 16, 2026]. doi:10.5455/JMID.2023.v13.i2.2 AMA (American Medical Association) Style Ibadin EE, Ogefere HO, Omoregie R, Igunma J. Prevalence of carbapenemase-producing organisms among patients admitted to intensive care unit in a tertiary hospital in Benin city, Nigeria. J Microbiol Infect Dis. 2023; 13(2): 53-58. doi:10.5455/JMID.2023.v13.i2.2 Vancouver/ICMJE Style Ibadin EE, Ogefere HO, Omoregie R, Igunma J. Prevalence of carbapenemase-producing organisms among patients admitted to intensive care unit in a tertiary hospital in Benin city, Nigeria. J Microbiol Infect Dis. (2023), [cited January 16, 2026]; 13(2): 53-58. doi:10.5455/JMID.2023.v13.i2.2 Harvard Style Ibadin, E. E., Ogefere, . H. O., Omoregie, . R. & Igunma, . J. (2023) Prevalence of carbapenemase-producing organisms among patients admitted to intensive care unit in a tertiary hospital in Benin city, Nigeria. J Microbiol Infect Dis, 13 (2), 53-58. doi:10.5455/JMID.2023.v13.i2.2 Turabian Style Ibadin, Ephraim E., Helen O. Ogefere, Richard Omoregie, and Jerry Igunma. 2023. Prevalence of carbapenemase-producing organisms among patients admitted to intensive care unit in a tertiary hospital in Benin city, Nigeria. Journal of Microbiology and Infectious Diseases, 13 (2), 53-58. doi:10.5455/JMID.2023.v13.i2.2 Chicago Style Ibadin, Ephraim E., Helen O. Ogefere, Richard Omoregie, and Jerry Igunma. "Prevalence of carbapenemase-producing organisms among patients admitted to intensive care unit in a tertiary hospital in Benin city, Nigeria." Journal of Microbiology and Infectious Diseases 13 (2023), 53-58. doi:10.5455/JMID.2023.v13.i2.2 MLA (The Modern Language Association) Style Ibadin, Ephraim E., Helen O. Ogefere, Richard Omoregie, and Jerry Igunma. "Prevalence of carbapenemase-producing organisms among patients admitted to intensive care unit in a tertiary hospital in Benin city, Nigeria." Journal of Microbiology and Infectious Diseases 13.2 (2023), 53-58. Print. doi:10.5455/JMID.2023.v13.i2.2 APA (American Psychological Association) Style Ibadin, E. E., Ogefere, . H. O., Omoregie, . R. & Igunma, . J. (2023) Prevalence of carbapenemase-producing organisms among patients admitted to intensive care unit in a tertiary hospital in Benin city, Nigeria. Journal of Microbiology and Infectious Diseases, 13 (2), 53-58. doi:10.5455/JMID.2023.v13.i2.2 |