| Review Article | ||
J Microbiol Infect Dis. 2023; 13(3): 98-109 J. Microbiol. Infect. Dis., (2023), Vol. 13(3): 098–109 Original Research Anti-quorum sensing effects of medicinal plants and chemical compounds: A new dimension against Pseudomonas aeruginosa and Staphylococcus aureusKingsley Chukwuemeka Nwachukwu1*, Ositadinma Chinyere Ugbogu1, Ijeoma Linda Nwosu2, and Ebubechukwu Nwarunma31Department of Microbiology, Abia State University, Uturu, Nigeria 2Department of Medical Laboratory Sciences, Abia State College of Health Sciences and Management Technology, Aba, Nigeria 3Department of Biological and Biomedical Science, Glasgow Caledonian University, Glasgow, Scotland *Corresponding Author: Marta Leiva. Servei d’Oftalmologia, Fundació Hospital Clínic Veterinari, Universitat Autònoma de Barcelona, Bellaterra, Spain. Email: marta.leiva [at] uab.cat Submitted: 06/11/2023 Accepted: 12/08/2023 Published: 30/09/2023 © 2023 Journal of Microbiology and Infectious Diseases
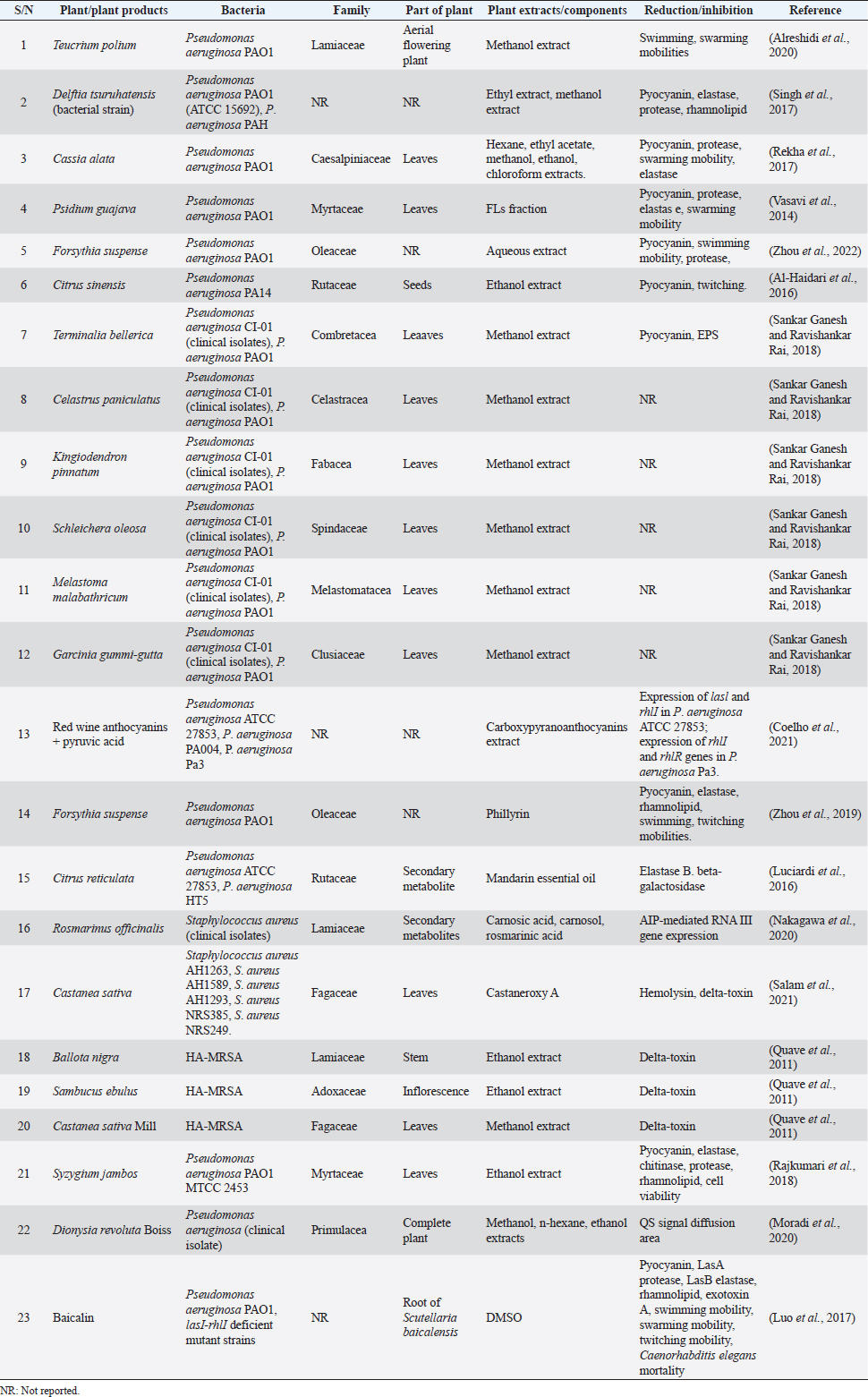
AbstractQuorum sensing (QS) is a communication mechanism utilized by microorganisms to deliberately unleash virulence factors for their survival in living or non-living hosts. Biofilm, one of the virulence factors that entrenches pathogenicity is developed through this process. Accumulation of biofilm as a result of heightened QS effect results in persistent spread of diseases and in extension, antibiotic resistance. Staphylococcus aureus and Pseudomonas aeruginosa are the major causative agents of nosocomial diseases which include wound and skin infections; so many synthetic and natural remedies have been introduced to reduce or entirely eliminate the development of QS-mediated virulence factors through repression of genes that encode them at the molecular level. Therefore, the activities of medicinal plants and chemical agents as inhibitors against QS-mediated virulence factors of P. aeruginosa and S. aureus strains are discussed and compared in this review. Overall, having the knowledge of target sites of plant extracts and chemical inhibitors and their mechanisms in these pathogenic bacterial cells as discussed in the review will assist scientists and medical practitioners in decision-making for better therapeutic regimens in the treatment of bacterial diseases. Keywords: Anti-quorum sensing, Medicinal plants, Drugs, Inhibitors. IntroductionTo prevent the problems of antibiotic resistance encountered during treatment, research is ongoing to discover new therapeutic agents that not only prevent bacterial growth but also, inhibit the expression of genes responsible for regulation of their virulence factors (Karathanasi et al., 2018)Click or tap here to enter text.. Different species of bacteria have been reported to produce stealth mechanisms that evade the effects of drugs; evidenced in the production of biofilm and expression of virulence factors through transcription of genes (Bouyahya et al., 2017). With these mechanisms, Pseudomonas aeruginosa and Staphylococcus aureus colonize the body of the host and inflict harm to vital organs, tissues, and cells. Staphylococcus aureus is a pathogenic microbe that mainly causes nosocomial infections in hospitalized patients. This Gram-positive bacterium utilizes the virulence factors in penetrating their hosts, decreasing their immune system and leading to the spread of diseases (Quave et al., 2015). The virulence factors responsible for pathogen-host interaction are expressed by the interaction of accessory gene regulators (agrB), agrD, agrC, and agrA accessory genes. These accessory genes which are activated by the P2 promoter, code for AgrB, AgrD, AgrC, and AgrA regulatory proteins, respectively. Upon stimulation by the P2 promoter, AgrB sends out a modified structure of AgrD to the external environment to release autoinducing peptides (AIPs) (Karathanasi et al., 2018). AIP attaches to AgrC to produce phosphate ions. The ion migrates to AgrA which consequently triggers the expression of RNAIII, an effector molecule through the control of the P3 promoter to release virulence factors especially, formed adherent cells and toxins (Schilcher and Horswill, 2020; Bernabè et al., 2021). Pseudomonas aeruginosa is a Gram-negative pathogenic bacterium commonly seen in patients with low immunity especially those with genetically related diseases, wounds, and catheter-influenced infections (Jakobsen et al., 2013). In P. aeruginosa, the expression of virulence factors depends on the effective interaction between the two major quorum sensing (QS) systems–las and rhl. Las and Rhl are protein receptors responsible for triggering autoinducers (AIs) synthesis. Lasl produces 3OC12-homoserine lactone (AI); at elevated density, 3OC12-HSL moves out of the cell and attaches to the LasR receptor (Vadakkan et al., 2018). The binding (LasR:3OC12-HSL) initiates the Rhl system. Rhl1 when synthesized by the transcription process produces a secondary AI (C4-homoserine lactone) that binds with RhlR to direct the transcription and regulation of genes. Apart from the initiation of the Rhl system, the LasR:3OC12-HSL complex also activates the production of virulence factors such as elastase, protease, and exotoxins while RhlR-C4-HSL produces pyocyanin and rhamnolipid virulence factors (Proctor et al., 2020). Initially, the las circuit has been the target of most drugs due to the obstruction of LasR, and was believed to reduce the activities of P. aeruginosa strains until recently when it was disproved. It was instead reported that in the absence of las system, RhlR was also able to activate precise LasR-controlled functions in P. aeruginosa including the LuxI/LuxR system (Saqr et al., 2021). This implied that one of the QS systems of P. aeruginosa can be active even in the absence of other systems. Hence, it is necessary to scout for new therapeutic agents that can target all the different levels of QS systems in bacteria and in turn decrease antibiotic resistance (Hemmati et al., 2020). Many inhibitory agents have been introduced to prevent the expression of the QS system (Başaran et al., 2020) and in extension, biofilm synthesis (Lu et al., 2021). Most medicinal plants and chemical products have proved effective in that regard (Tables 1 and 2). Therefore, the activities of medicinal plants, their bioactive metabolites, and chemical agents or drugs as inhibitors against QS-mediated virulence factors and biofilms of P. aeruginosa and S. aureus strains were discussed and compared in this review. Plant as QS inhibitors in P. aeruginosaMedicinal plant extracts interact and alter the regulation of Pqs Rhl and Las genes in the QS system, responsible for encoding virulence factors and promulgation of pathogenicity in strains of P. aeruginosa (Yang et al., 2020). When promoter sites in these strains are targeted by biomolecules from the plant extracts, genes that encode the proteins could either be down-regulated or up-regulated. In an experiment to determine the efficacy of flavonoids (FLs), a secondary metabolite against QS of P. aeruginosa, Paczkowski et al. (2017) observed that FLs prevented LasR a QS receptor from attaching the DNA molecule through the allosteric binding mechanism. The inability of the metabolite to displace AI from its binding site makes it a perfect anti-QS agent compared to other bioactive molecules. The researchers demonstrated that FLs are good inhibitors of the QS receptor (LasR) and that they do not function via a competitive mechanism that involves the displacement of the natural AIs from their binding pocket. Rather, the FLs bind to the LasR and prevent the protein from binding to DNA leading to disruption of virulence factors. Luciardi et al. (2016) investigated QS inhibition of Mandarin essential oil. The researchers prepared the essential oil by cold-pressing (EOP) and cold-pressing followed by distillation (EOPD). At 4 mg/ml, both extracts did not produce any significant effects on the growth of P. aeruginosa ATCC 783 (33.8%) and P. aeruginosa HT5 (25%); however, at the same concentration, 3OC12-HSL production was reduced by 33% and 75%, respectively. Several authors: Rajkumari et al. (2018), Sankar Ganesh and Ravishankar Rai (2018), Ahmed et al. (2019), Singh et al. (2017), and Vasavi et al. (2014) in their separate studies investigated the activities of medicinal plants on QS-mediated virulence factors in P. aeruginosa PAO1. Rajkumari et al. (2018) Click or tap here to enter text. discovered that at sub-MIC of 500 μg/ml, Syzygium jambos (L) Alston extract reduced pyocyanin, elastase, protease, and chitinase enzymes by 74.59%, 24. 94%, 80.96%, and 69.52%, respectively. The most bioactive extracts (n-butanol, dichloromethane, hexane, and ethyl acetate) were chosen after subjecting them to molecular docking against the QS receptor of P. aeruginosa PAO1. In a related study, Sankar Ganesh and Ravishankar Rai (2018) investigated the anti-QS of six medicinal plants (Terminalia bellerica, Celastrus paniculanrus, Kingiodendrin pinnatum, Schleicheta oleosa, Melastoma malabathricum, and Gorcinia gummi-gutta). The methanol extract of T. bellerica was the most effective bioactive extract among them and was tested for its inhibitory effects on exopolysaccharides (EPSs) and pyocyanin production. At 0.5 and 1 mg/ml, the extract produced 97.47% and 42% reduction, respectively, while 18.55%, 27.83%, 54.71%, and 67.99% of EPS reduction resulted from 0.0625, 0.125 to 0.5 mg/ml of T. bellerca methanol extract, respectively. Zhou et al. (2019) studied the anti-QS effect of Phillyrin on QS-mediated virulence factors at different concentrations. Phillyrin is a secondary metabolite obtained from Forsythia suspense (Thunb) Vahl, one of the major constituents of Chinese herbal drugs known for its anti-inflammatory (Sung et al., 2016) and antimicrobial (Zhou et al., 2022) activities. At 0.25, 0.125, and 0.0625 mg/ml, phillyrin reduced pyocyanin by 85.94%, 65.16%, and 50.17%, respectively. Between the ranges of 0.0625–0.25 mg/ml, elastase was reduced by 35.53%, 65.68%, and 89.95%, respectively. Swimming zone was reduced from 67 ± 4.3 (control) to 13.3 ± 3.8 mm; for twitching mobility, it was reduced from 54 ± 2.8 (control) to 11 ± 2.11 mm at 0.25 mg/ml. Vasavi et al. (2014) obtained an FL fraction of Psidium guajava methanol extract (25 to 400 μg/ml) and tested it against QS-mediated virulence factors in P. aeruginosa PAO1. Pyocyanin, elastase and protease production, and swimming mobility were all determined. At 25 μg/ml, FL-fraction reduced pyocyanin production by 50%. There was complete inhibition (100%) of swimming mobility at 25 μg/ml; elastase and protease enzyme production at 200 μg/ml. Zhang and Chu (2017) went ahead to monitor the anti-QS effects of aqueous extract of F. suspense on C. violaceum ATCC 12472 (reference strain) but investigated its activity on QS-mediated virulence factors in P. aeruginosa PAOI. They worked only on the inhibitory effects of the extracts against elastase and pyocyanin production and swimming mobility of the bacteria. Elastase from the study was reduced by 40.97%, pyocyanin had a 47.58% reduction while swimming mobility was reduced completely (100%) at 0.25 μg/ml. Table 1. Common medicinal plants examined as anti-QS agents against P. aeruginosa and S. aureus strains.
Plant as QS inhibitors in S. aureusNakagawa et al. (2020) examined the effects of carnosic acid, carnosol, and rosmarinic acid obtained from Rosmarinus officianis (Rosemary) on AIP-mediated agr gene and psmα gene virulence expressions in S. aureus. Nine different extracts of R. officianis with various concentrations of carnosic acid, carnosol, and rosmarinic acid were used for the study. All the extract groups (C1-C9) at 5 μg/ml, repressed significantly, AIP-mediated agr gene expression except the third group (C3). At 10 μg/ml, the carnosic acid molecule inhibited AIP-mediated RNAIII gene expression while the carnosol molecule produced an appreciable inhibition at a low concentration of 5 μg/ml. Both molecules inhibited AIP-mediated psmα gene virulence expressions at 10 μg/ml. Sharifi et al. (2018) studied the anti-QS activities of Thymus daenensis and Satureja hortensis EOs at the molecular level using a quantitative real-time polymerase chain reaction (PCR) assay. It was discovered from the result that at 0.0625 μl/ml, S. hortensis decreased the expression of hld genes while at 0.0312 μl/ml, no observable change was seen in hld genes expression of S. aureus when treated with other EOs. The researchers therefore posited that EO of S. hortensis was an effective anti-QS agent against S. aureus. Quave et al. (2011) investigated agr system in healthcare-associated methicillin-resistant Staphylococcus aureus (HA-MRSA) by quantifying delta-toxin production using HPLC. In their study, it was revealed that at 64 μg/ml concentrations, only ethanol extracts of Ballota nigra, Castanea sativa, and Sambucus ebulus produced significant inhibition of delta-toxin inhibited delta toxin in HA-MRSA out of 168 plant extracts (from 104 plants). In 2015, Quave et al. (2015) extended their investigation with leaves of C. sativa (European chestnut). They explored the activities of three fractions: 224, 224C, and 224C-F2 of C. sativa on reporter strains of S. aureus. Among these fractions, 224C-F2 (obtained from 224C fraction after flash chromatography system using hexane, ethyl acetate, and methanol) produced significant anti-QS effects against agr P3-YFP four reporter strains. It inhibited delta-toxin (0.25 μg/ml; p < 0.01) as well as alpha-hemolysin synthesis in wild-type (6.25 μg/ml; p < 0.001) and mutant strains. At IC50 values of 1.56 μg/ml, 224C-F2 fraction produced the highest anti-QS activity in the AH1747 strain while at 25 μg/ml in AH1872, the fraction synthesized the lowest anti-QS effect. From in vivo study carried out in mice, it was discovered that 224C-F2 was not harmful to human immortalized keratinocytes (HaCaT cells) at 512 μg/ml and their skin structure at 5 and 10 μg/ml after quantification. The researchers, therefore, posited that apart from ursene and oleanene which are major constituents of C. sativa, other biomolecules such as gallotannins, and ellagitannin were identified and are responsible for its anti-QS effects against strains of S. aureus. The extracts were also safe for skin application. Table 2. Chemical agents/drugs used as anti-QS agents against P. aeruginosa and S. aureus strains.
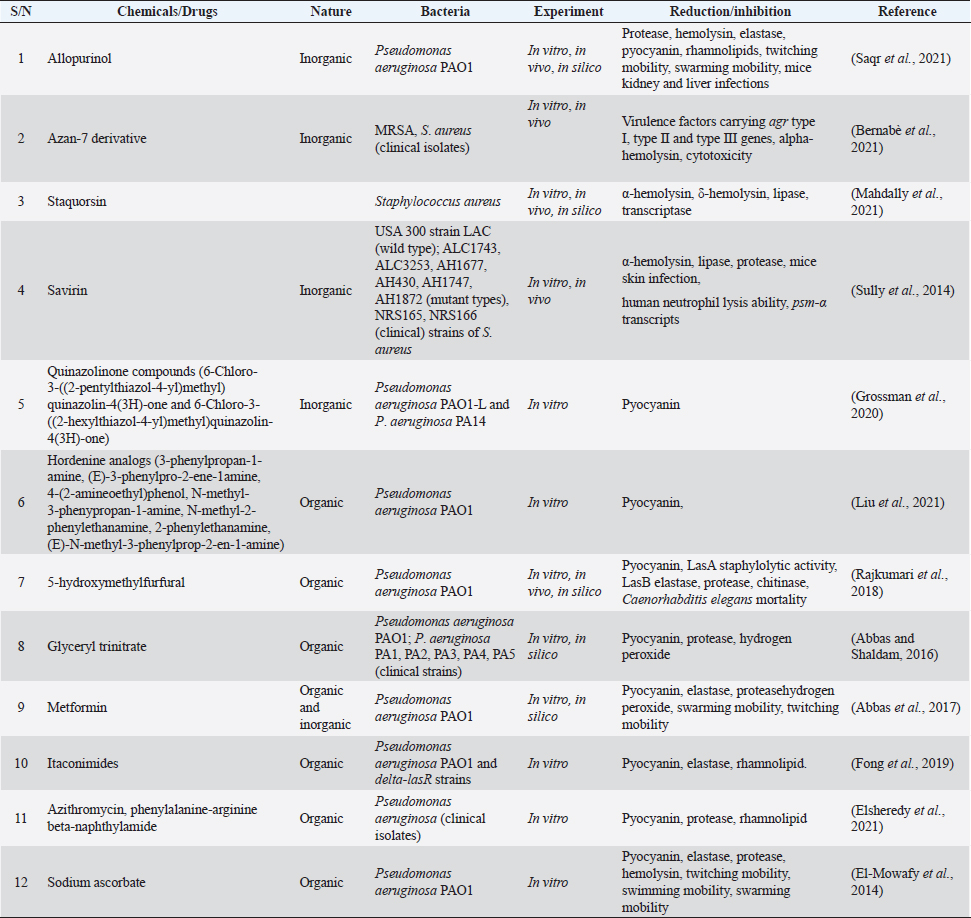
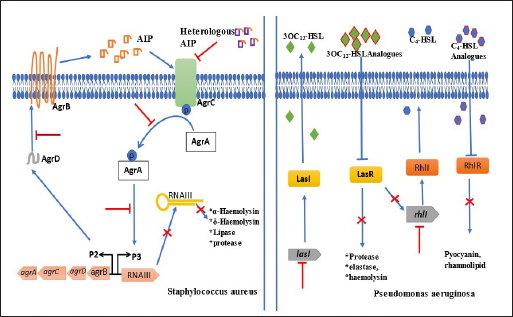
Chemicals as QS inhibitors in P. aeruginosaGrossman et al. (2020) examined the anti-QS effects of quinazoline on PqsR, a transcriptional enzyme regulating pqs QS system in P. aeruginosa PAO1-L and P. aeruginosa PA14. It was revealed that at 3 × IC50 value, 6-Chloro-3-((2-pentylthiazol-4-yl)methyl)quinazolin-4(3H)-one and 6-Chloro-3-((2-hexylthiazol-4-yl)methyl)quinazolin-4(3H)-one among other chemicals reduced pyocyanin production by 23% and 36%, respectively in P. aeruginosa PAO1-L. The compounds were not toxic to lung epithelial cells at the highest concentration of 100 μM. After insertion of the bioluminescence-producing gene (Mctx:PpqsA-lux) into the chromosome of PAO1-L and PA14 strains, bioluminescence was significantly reduced to more than 50% by 14 chemicals mainly alkylated quinazolines at 10 μM. The authors suggested that the appreciable inhibition of pyocyanin synthesis with no toxicity was a result of chlorine substitute at quinazolinone structure in the compounds. Saqr et al. (2021) studied allopurinol as an anti-QS agent against P. aeruginosa PAO1. From the in silico study, allopurinol competed with 3OC12-HSL and C4-HSL AIs for LasR and RhlR receptors, respectively, while in a molecular study conducted with qRT-PCR, it was discovered that the drug downregulated rhlI, rhlR, lasI, lasR, pqsA, and pqsR genes responsible for encoding QS enzymes. Allopurinol, at 1/10 MIC of 200 μg/ml reduced hemolysin and pyocyanin quantified with spectrophotometer (691 nm) by 95% and 74%, respectively; elastase production was reduced by 93% at 1/10 MIC. Protease, synthesized with skim milk agar method had a reduction of 55% at 1/10 MIC while rhamnolipid production (oil displacement method) at 1/10 MIC was reduced by 74%. At the same sub-MIC, the twitching and swarming abilities of the strain were reduced appreciably (p < 0.001) by 92%, 87%, and 85%, respectively. Glyceryl trinitrate, a chemical substance that cures high blood pressure, was recently found to possess antibacterial activity. The anti-QS effect of glyceryl trinitrate on five clinical strains (PA1, PA2, PA3, PA4, and PA5) from wound infections and the PAO1 strain of P. aeruginosa was investigated by Abbas and Shaldam (2016) the highest values between 41.28% and 3.52%. The researchers posited that the EO of S. hortensis was an effective anti-QS agent against S. aureus. Quave et al. (2011) investigated agr system by quantifying delta-toxin production in HA-MRSA using HPLC. In the study, it was revealed that between 8 and 256 μg/ml concentrations, only ethanol extracts of Ballota nigra, C. sativa, and S. ebulus inhibited delta toxin in HA-MRSA out of 168 plant extracts (from 104 plants). Furthermore, Abbas et al. (2017) investigated the potential of metformin in inhibiting QS in P. aeruginosa strains. Their investigation centered on the effects of the agent on protease, pyocyanin, elastase, and hemolysin production as well as swimming and twitching mobilities. Protease production was reduced by 21.48%, pyocyanin was reduced by 48.67%, elastase had a 23.26% reduction while the hemolytic activity of PAO1 was reduced by 54.27%. Further analysis revealed that the swimming and twitching mobilities were reduced by 46.78% and 58%, respectively. From the result of molecular docking activity, the researchers posited, therefore, that the inhibition of QS activity of metformin resulted from the interaction with LasR and RhlR receptors. The QS inhibitory activity of Itaconimides on elastase, rhamnolipid, and pyocyanin virulence factors synthesis in P. aeruginosa (lasB-gfp) strain was examined by Fong et al. (2019), as well as the cytotoxic effects. At 10 μM, both p-bromophenyl and tetradecyl-substituted itaconimides reduced elastase production by half and total elimination (100%) of pyocyanin and rhamnolipid synthesis. The gene responsible for encoding these virulence factors was repressed in a dose-dependent method. To exactly detect which genes were targeted by the chemical agents, P. aeruginosa PAO1 and delta-lasR strains that possess rhlA-gfp and pqsA-gfp genes were investigated. The p-bromophenyl-substituted itaconimides inhibited rhlA-gfp gene expression by 78% in P. aeruginosa delta-lasR strain and 58% in P. aeruginosa PAO1 while inhibition took place toward the ending stage for pqsA-gfp genes in P. aeruginosa delta-lasR strain. The expression of rhlA-gfp and pqsA-gfp genes was completely inhibited in P. aeruginosa delta-lasR strain by tetradecyl-substituted itaconimides. From the outcome of gene expression inhibition processes, it was suggested that tetradecyl-substituted itaconimides were more effective than the other derivatives as they attacked several QS pathways. The cytotoxicity assay showed that the compounds did not produce toxicity even at 40 μM concentration with little effect on macrophages. Chemical agent as QS inhibitors in S. aureusBernabè et al. (2021) investigated the effect of Aza derivatives as an anti-QS agent against MRSA. Their investigation included a QS-mediated virulence factor inhibition assay, growth inhibition assay, cytotoxic assay, and hemolysin inhibition assay. In a bid to determine the most effective derivatives, the researchers grew MRSA separately with all 16 derivatives of Azan compounds in 0.05% DMSO (control) and quantified them at 600 nm. It was seen that after 16 hours, Azan-3, Azan-2, Azan-6, Azan-7, and Azan-10 inhibited rna 3 from expressing virulence factors however, only Azan-7 was more potent. To determine the actual concentration needed by Azan-7, MRSA was grown in LB broth at 100 μM, 50 μM, and 10 μM. Azan-7, at 100 μM reduced maximally hla, psmα, hysA, agrA, cap1A, and cap1C gene expression responsible for virulence factors. After culturing MRSA with Azan-7, it was discovered that α-hemolysin was reduced in MRSA and it did not cause the release of a large quantity of hemoglobin from the red blood cells of rats after 16 hours. No in silico docking was performed. Mahdally et al. (2021), reported effect of staquorsin (4-Methoxy-N′-(phthalazin-1-yl) benzenesulfonohydrazide hydrochloride) on Agr-regulated QS in S. aureus in an in vivo study. α-hemolysin, δ-hemolysin, and lipase inhibition assays were determined as well as cytotoxicity and in silico molecular docking assays. Staquorsin reduced α-hemolysin in S. aureus (wild type) to nearly in large extent with mutant strain (delta agrB). δ-hemolysin was reduced by 80% ( p < 0.0001) which was slightly lower than delta agrB while the lipase production was reduced by 50% ( p < 0.0001). After discovering that the mammalian cell lines were unaffected even at 40 μM, the researchers proceeded by conducting in vivo study with staquorsin and comparing it with the control agent (savarin). It was realized that mice treated with 40 nmol of staquorsin in 50 μl of 0.5% hydroxypropyl methylcellulose added weight and experienced healing from ulcer and bacterial skin infection while savarin produced the direct opposite. Staquorsin bound perfectly at the active site through H-bonding with Arg218 and Val232 with a −6.67 kcal/mol docking score. Moreover, even after several dilutions of S. aureus treated with the agent, QS-mediated virulence factors were still reduced significantly. Targets of QS inhibitors in P. aeruginosa and S. aureusBoth medicinal plants or their products and chemical agents target at specific locations, the binding of AIs to receptors or degrade their synthesis while some, consistently target the expression of genes responsible for the development of virulence factors (Asfour, 2018; Paluch et al., 2020). There are three QS pathways in P. aeruginosa, namely las, rhl, and pqs systems. Whereas las and rhl systems utilized 3OC12-HSL and C4-HSL AIs, respectively (Paluch et al., 2020), pqs system relied on 2-alkyl-4(1H)-quinolone for effective communication (Yang et al., 2020). The LasI and RhlI proteins trigger the synthesis of 3OC12-HSL and C4-HSL which in turn bind to LasR and RhlR receptors, respectively. The binding (LasR:3OC12-HSL) promotes the transcription of lasI and lasR genes that encode the LasI synthase and LasR receptor. LasR:3OC12-HSL and RhlI:C4-HSL trigger the expression of rhlI that encodes RhlI synthase and rhlR that encodes RhlR receptor. LasR:3OC12-HSL further promotes the pqsR gene while RhlI:C4-HSL complex decreases the transcription of the gene. The pqs and rhl systems are controlled by the las system; thus, making it assume the highest place in the ladder of QS activities in the bacterial cell. In this review, we looked mainly at las and rhl systems (Fig. 1) because they control the expression of many genes including those responsible for virulence factors (Alfiniyah et al., 2019), and the targets of inhibitors were focused only on the two of them. The LasR and RhlI receptors are unarguably, the targets of most QS inhibitors. The attack on LasR and RhlI terminates the effective signaling pathway in the cell and leads to a decline in the expression of virulence factors. The chemical compound 2-(3-(Naphthalen-1-yl)ureido)-2-oxoethyl(S)-(2-oxotetrahydrofuran-3-yl)carbamodith-ioate, being the analogue of 3OC12-HSL targeted LasR by outcompeting it. It prevented the transcription of lasR and rhlR genes in the las system. From the docking experiment with PAO1-JP2, Markus et al. (2021) discovered that furanone C-30 (synthetic agent) targeted both LasR and RhlR receptors separately but directly, at their active sites. In another docking study performed by Muciño (2022), eugenol, hydrocinnamic acid, and ferulic acid were discovered to target and bind to the RhlR receptor; however, following the Binding Gibbs energy test performed further by the researchers, only eugenol and ferulic acid appeared to be more stable. FLs inhibited the expression of virulence genes by attacking the LasR receptor through the allosteric process (Paczkowski et al., 2017). Naringenin, another FL known for its healing effect, binds to LasR partially at the same site as the natural ligands; its efficacy hinged on the period of attack of the receptor (Hernando-Amado et al., 2020) Zhao et al. (2021) confirmed that falcarindol, a natural compound obtained from Notopterygium incisum targeted also LasR receptor. Luo et al. (2017) in their antibiofilm study, confirmed that baicalin attacked the biofilm of P. aeruginosa cells at the point of colonization to surface, aggregation, and growth. In S. aureus, accessory gene regulator (agr) system is usually the signaling pathway often utilized in the synthesis of AIP (Wang and Muir, 2016) and regulation of virulence genes and cell adhesion proteins (Traber et al., 2008). After activation by the P3 promoter, the RNAIII, an effector molecule of agr system coordinates the release of virulence factors especially exotoxins (Gong et al., 2014). Other Qs regulatory systems that enhance the pathogenicity in S. aureus are luxS (Le and Otto, 2015) and sar (Ganesh et al., 2022) systems. However, due to the overriding importance of the agr system, this review focused only on it (Fig. 1). In the cells of S. aureus strains, the targets of the inhibitors are AIP: AgrC complex, which could be by modification of AIPs amino acid structure, elimination of exocyclic appendage and polymerization of the AIPs (Le and Otto, 2015). The inhibitors also could block the binding of AgrA protein to P3 and AgrD. Karathanasi et al. (2018) confirmed that in some strains of S. aureus, linear peptidomimetics, and AIP analogs attacked the AIP: AgrC integration. Moreover, the binding of AgrA protein to P3 was the target of Azan-7 derivatives (Bernabè et al., 2021), Savirin (Sully et al., 2014), Staquorsin (Mahdally et al., 2021). Baicalein eliminated the biofilm formed at the dispersive stage as a result of a direct attack of AgrD, preventing it from binding to AgrC. It was also suggested that this natural agent targeted the RNAIII activating molecule (Chen et al., 2016).
Fig. 1. Schematic diagram demonstrating targets and effects of QS inhibitors in S. aureus and P. aeruginosa. ConclusionDifferent strains of P. aeruginosa and S. aureus produced biofilm and virulence factors regulated at the molecular levels. The genes that encode for the synthesis of pyocyanin, protease, exotoxin, hemolysin, elastase, lipase, and rhamnolipid enzymes, as well as twitching, swimming, and swarming mobilities, were repressed by both plant extracts and synthetic drugs. However, these activities were dependent on the nature of bioactive components in plants and active ingredients of chemical compounds. From the review, comparing medicinal plants and other plant components with chemicals or drugs, plant extracts and essential oils performed better in producing efficient inhibition of both biofilm and QS. Between the bacterial species, P. aeruginosa PAO1 and MRSA were consistently studied by most authors, an indication that these strains have caused untold pain to patients and should be a serious concern to health care providers. This review opens up a new era of herbal therapy in the treatment of diseases especially skin and hospital-related infections, since they produced limited or no side effects when compared with synthetic drugs. ReferencesAbbas, H.A., Elsherbini, A.M. and Shaldam, M.A. 2017. Repurposing metformin as a quorum sensing inhibitor in Pseudomonas aeruginosa. Afr. Health. Sci. 17(3), 808–819. Abbas, H.A.M.A.H. and Shaldam, M.A. 2016. Glyceryl trinitrate is a novel inhibitor of quorum sensing in Pseudomonas aeruginosa. Afr. Health. Sci. 16(4), 1109–1117. Ahmed, S.A.K.S., Rudden, M., Smyth, T.J., Dooley, J.S.G., Marchant, R. and Banat, I.M. 2019. Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl. Microbiol. Biotechnol. 103, 3521–3535. Alfiniyah, C., Bees, M.A. and Wood, A.J. 2019. Quorum machinery: effect of the las system in rhl regulation of P. aeruginosa. In AIP Conference Proceedings. American Institute of Physics Inc., 2019. Al-Haidari, R.A., Shaaban, M.I., Ibrahim, S.R.M. and Mohamed, G.A. 2016. Anti-quorum sensing activity of some medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 13(5), 67–71. Alreshidi, M., Noumi, E., Bouslama, L., Ceylan, O., Veettil, V.N., Adnan, M., Danciu, C., Elkahoui, S., Badraoui, R., Al-Motair, K.A. and Patel, M. 2020. Phytochemical screening, antibacterial, antifungal, antiviral, cytotoxic, and anti-quorum-sensing properties of Teucrium polium l. aerial parts methanolic extract. Plants 9(11), 1–20. Asfour, H. 2018. Anti-quorum sensing natural compounds. J. Microsc. Ultrastruct. 6(1), 1. Başaran, T.I., Berber, D., Gökalsın, B., Tramice, A., Tommonaro, G., Abbamondi, G.R., Erginer Hasköylü, M., Toksoy Öner, E., Iodice, C. and Sesal, N.C. 2020. Extremophilic Natrinema versiforme against Pseudomonas aeruginosa quorum sensing and biofilm. Front. Microbiol. 11, 79. Bernabè, G., Dal Pra, M., Ronca, V., Pauletto, A., Marzaro, G., Saluzzo, F., Stefani, A., Artusi, I., De Filippis, V., Ferlin, M.G. and Brun, P. 2021. A novel aza-derivative inhibits agr quorum sensing signaling and synergizes methicillin-resistant Staphylococcus aureus to clindamycin. Front. Microbiol. 12, 610859. Bouyahya, A., Dakka, N., Et-Touys, A., Abrini, J. and Bakri, Y. 2017. Medicinal plant products targeting quorum sensing for combating bacterial infections. Asian. Pac. J. Trop. Med. 10, 729–743. Chen, Y., Liu, T., Wang, K.E., Hou, C., Cai, S., Huang, Y., Du, Z., Huang, H., Kong, J. and Chen, Y. 2016. Baicalein inhibits Staphylococcus aureus biofilm formation and the quorum sensing system in vitro. PLoS One 11(4), e0153468. Coelho, P., Oliveira, J., Fernandes, I., Araújo, P., Pereira, A.R., Gameiro, P. and Bessa, L.J. 2021. Pyranoanthocyanins interfering with the quorum sensing of Pseudomonas aeruginosa and Staphylococcus aureus. Int. J. Mol. Sci. 22(16), 8559. El-Mowafy, S.A., Shaaban, M.I., Abd, E.L. and Galil, K.H. 2014. Sodium ascorbate as a quorum sensing inhibitor of Pseudomonas aeruginosa. J. Appl. Microbiol. 117(5), 1388–1399. Elsheredy, A., El-Soudany, I., Elsherbini, E., Metwally, D. and Ghazal, A. 2021. Effect of azithromycin and phenylalanine-arginine beta-naphthylamide on quorum sensing and virulence factors in clinical isolates of Pseudomonas aeruginosa [Internet]. Iran. J. Microbiol. 13(1), 37. Available via http://ijm.tums.ac.ir Fong, J., Mortensen, K.T., Nørskov, A., Qvortrup, K., Yang, L., Tan, C.H., Nielsen, T.E. and Givskov, M. 2019. Itaconimides as novel quorum sensing inhibitors of Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 9(JAN), 443. Ganesh, P.S., Veena, K., Senthil, R., Iswamy, K., Ponmalar, E.M., Mariappan, V., Girija, A.S., Vadivelu, J., Nagarajan, S., Challabathula, D. and Shankar, E.M. 2022. Biofilm-associated agr and sar quorum sensing systems of Staphylococcus aureus are inhibited by 3-hydroxybenzoic acid derived from Illicium verum. ACS. Omega. 7, 14653–14665. Available via https://doi.org/10.1021/acsomega.1c07178 Gong, J., Li, D., Yan, J., Liu, Y., Li, D., Dong, J., Gao, Y., Sun, T. and Yang, G. 2014. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine intracranial abscesses model. Braz. J. Infect. Dis. 18(5), 501–506. Grossman, S., Soukarieh, F., Richardson, W., Liu, R., Mashabi, A., Emsley, J., Williams, P., Cámara, M. and Stocks, M.J. 2020. Novel quinazolinone inhibitors of the Pseudomonas aeruginosa quorum sensing transcriptional regulator PqsR. Eur. J. Med. Chem. 208, 112778. Hemmati, F., Salehi, R., Ghotaslou, R., Samadi Kafil, H., Hasani, A., Gholizadeh, P., Nouri, R. and Ahangarzadeh Rezaee, M. 2020. Quorum quenching: a potential target for antipseudomonal therapy. Infect. Drug. Resist. 13, 2989–3005. Hernando-Amado, S., Alcalde-Rico, M., Gil-Gil, T., Valverde, J.R. and Martínez, J.L. 2020. Naringenin inhibition of the Pseudomonas aeruginosa quorum sensing response is based on its time-dependent competition with N-(3-Oxo-dodecanoyl)-L-homoserine lactone for LasR binding. Front. Mol. Biosci. 7, 25. Jakobsen, T.H., Bjarnsholt, T., Jensen, P.O., Givskov, M. and Høiby, N. 2013. Targeting quorum sensing in Pseudomonas aeruginosa biofilms: current and emerging inhibitors. Future. Microbiol. 8, 901–921. Karathanasi, G., Bojer, M.S., Baldry, M., Johannessen, B.A., Wolff, S., Greco, I., Kilstrup, M., Hansen, P.R. and Ingmer, H. 2018. Linear peptidomimetics as potent antagonists of Staphylococcus aureus agr quorum sensing. Sci. Rep. 8(1), 3562. Le, K. and Otto, M. 2015. Quorum-sensing regulation in staphylococci-an overview. Front. Microbiol. [Internet]. 6, 1174. Available via www.frontiersin.org Liu, Y., Li, J.J., Li, H.Y., Deng, S.M. and Jia, A.Q. 2021. Quorum sensing inhibition of hordenine analogs on Pseudomonas aeruginosa and Serratia marcescens. Synth. Syst. Biotechnol. 6(4), 360–368. Lu, L., Zhao, Y., Yi, G., Li, M., Liao, L., Yang, C., Cho, C., Zhang, B., Zhu, J., Zou, K. and Cheng, Q. 2021. Quinic acid: a potential antibiofilm agent against clinical resistant Pseudomonas aeruginosa. Chin. Med. (United Kingdom). 16(1), 1–17. Luciardi, M.C., Blázquez, M.A., Cartagena, E., Bardón, A. and Arena, M.E. 2016. Mandarin essential oils inhibit quorum sensing and virulence factors of Pseudomonas aeruginosa. LWT. Food. Sci. Technol. 68, 373–380. Luo, J., Dong, B., Wang, K., Cai, S., Liu, T., Cheng, X., Lei, D., Chen, Y., Li, Y., Kong, J. and Chen, Y. 2017. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS One 12(4), e0176883. Mahdally, N.H., George, R.F., Kashef, M.T., Al-Ghobashy, M., Murad, F.E. and Attia, A.S. 2021. Staquorsin: a novel Staphylococcus aureus agr-mediated quorum sensing inhibitor impairing virulence in vivo without notable resistance development. Front. Microbiol. 12, 700494. Markus, V., Golberg, K., Teralı, K., Ozer, N., Kramarsky-Winter, E., Marks, R.S. and Kushmaro, A. 2021. Assessing the molecular targets and mode of action of furanone c-30 on pseudomonas aeruginosa quorum sensing. Molecules 26(6), 1620. Moradi, F., Hadi, N. and Bazargani, A. 2020. Evaluation of quorum-sensing inhibitory effects of extracts of three traditional medicine plants with known antibacterial properties. New. Microbes. New. Infect. 38, 100769. Muciño, E.E. 2022. The role of eugenol and ferulic acid as the competitive inhibitors of transcriptional regulator RhlR in P. aeruginosa. MethodsX 9, 101771. Nakagawa, S., Hillebrand, G.G. and Nunez, G. 2020. Rosmarinus officinalis l. (rosemary) extracts containing carnosic acid and carnosol are potent quorum sensing inhibitors of Staphylococcus aureus virulence. Antibiotics 9(4), 149. Paczkowski, J.E., Mukherjee, S., McCready, A.R., Cong, J.P., Aquino, C.J., Kim, H., Henke, B.R., Smith, C.D. and Bassler, B.L. 2017. Flavonoids suppress Pseudomonas aeruginosa virulence through allosteric inhibition of quorum-sensing receptors. J. Biol. Chem. 292(10),4064–4076. Paluch, E., Rewak-Soroczyńska, J., Jędrusik, I., Mazurkiewicz. E. and Jermakow, K. 2020. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 104, 1871–1881. Proctor, C.R., McCarron, P.A. and Ternan, N.G. 2020. Furanone quorum-sensing inhibitors with potential as novel therapeutics against Pseudomonas aeruginosa. J. Med. Microbiol. 69, 195–206. Quave, C.L., Lyles, J.T., Kavanaugh, J.S., Nelson, K., Parlet, C.P., Crosby, H.A., Heilmann, K.P. and Horswill, A.R. 2015. Castanea sativa (European Chestnut) leaf extracts rich in ursene and oleanene derivatives block Staphylococcus aureus virulence and pathogenesis without detectable resistance. PLoS One 10(8), e0136486. Quave, C.L., Plano, L.R.W. and Bennett, B.C. 2011. Quorum sensing inhibitors of Staphylococcus aureus from Italian medicinal plants. Planta. Med. 77(2):188–195. Rajkumari, J., Borkotoky, S., Murali, A. and Busi, S. 2018. Anti-quorum sensing activity of Syzygium jambos (L.) Alston against Pseudomonas aeruginosa PAO1 and identification of its bioactive components. S. Afr. J. Bot. 118, 151–157. Rekha, P.D., Vasavi, H.S., Vipin, C., Saptami, K. and Arun, A.B. 2017. A medicinal herb Cassia alata attenuates quorum sensing in Chromobacterium violaceum and Pseudomonas aeruginosa. Lett. Appl. Microbiol. 64(3), 231–238. Salam, A.M., Porras, G., Cho, Y.S.K., Brown, M.M., Risener, C.J., Marquez, L., Lyles, J.T., Bacsa, J., Horswill, A.R. and Quave, C.L. 2021. Castaneroxy A from the leaves of Castanea sativa inhibits virulence in Staphylococcus aureus. Front. Pharmacol. 12, 640179. Sankar Ganesh, P. and Ravishankar Rai, V. 2018. Attenuation of quorum-sensing-dependent virulence factors and biofilm formation by medicinal plants against antibiotic resistant Pseudomonas aeruginosa. J. Tradit. Complement. Med. 8(1), 170–177. Saqr, A.A., Aldawsari, M.F., Khafagy, E.S., Shaldam, M.A., Hegazy, W.A.H. and Abbas, H.A. 2021. A novel use of allopurinol as a quorum-sensing inhibitor in Pseudomonas aeruginosa. Antibiotics 10(11), 1385. Schilcher, K. and Horswill, A.R. 2020. Staphylococcal biofilm development: structure, regulation, and treatment strategies [Internet]. Microbiol. Mol. Biol. Rev. 84(3), 10–1128. Available via https://journals.asm.org/journal/mmbr Sharifi, A., Mohammadzadeh, A., Zahraei Salehi, T. and Mahmoodi, P. 2018. Antibacterial, antibiofilm and antiquorum sensing effects of Thymus daenensis and Satureja hortensis essential oils against Staphylococcus aureus isolates. J. Appl. Microbiol. 124(2), 379–388. Singh, V.K., Mishra, A. and Jha, B. 2017. Anti-quorum sensing and anti-biofilm activity of Delftia tsuruhatensis extract by attenuating the quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 7, 337. Sully, E.K., Malachowa, N., Elmore, B.O., Alexander, S.M., Femling, J.K., Gray, B.M., DeLeo, F.R., Otto, M., Cheung, A.L., Edwards, B.S. and Sklar, L.A. 2014. Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS. Pathog. 10(6), e1004174. Sung, Y.Y., Yoon, T., Jang, S. and Kyoung Kim, H. 2016. Forsythia suspensa suppresses house dust mite extract-induced atopic dermatitis in NC/Nga mice. PloS One 11(12), e0167687. Traber, K.E., Lee, E., Benson, S., Corrigan, R., Cantera, M., Shopsin, B. and Novick, R.P. 2008. Agr function in clinical Staphylococcus aureus isolates. Microbiology (N Y) 154(8), 2265–2274. Vadakkan, K., Choudhury, A.A., Gunasekaran, R., Hemapriya, J. and Vijayanand, S. 2018. Quorum sensing intervened bacterial signaling: pursuit of its cognizance and repression. J. Genetic. Eng. Biotechnol. 16(2), 239–252. Vasavi, H.S., Arun, A.B. and Rekha, P.D. 2014. Anti-quorum sensing activity of Psidium guajava L. flavonoids against Chromobacterium violaceum and Pseudomonas aeruginosa PAO1. Microbiol. Immunol. 58(5), 286–293. Wang, B. and Muir, T.W. 2016. Regulation of virulence in Staphylococcus aureus: molecular mechanisms and remaining puzzles. Cell. Chem. Biol. 23, 214–224. Yang, W., Wei, Q., Tong, Q., Cui, K., He, G., Lin, L., Ma, L.Z., Cornelis, P. and Wang, Y. 2020. Traditional Chinese medicine tanreqing inhibits quorum sensing systems in Pseudomonas aeruginosa. Front. Microbiol. [Internet]. 11, 517462. Available via www.frontiersin.org Zhang, A. and Chu, W.H. 2017. Anti-quorum sensing activity of Forsythia suspense on Chromobacterium violaceum and Pseudomonas aeruginosa. Pharmacogn. Mag. 13(50), 321–325. Zhou, C., Lu, M., Cheng, J., Rohani, E.R., Hamezah, H.S., Han, R. and Tong, X. 2022. review on the pharmacological properties of Phillyrin. Molecules 27(12), 3670; https://doi.org/10.3390/molecules27123670 Zhou, S., Zhang, A. and Chu, W. 2019. Phillyrin is an effective inhibitor of quorum sensing with potential as an anti-Pseudomonas aeruginosa infection therapy. J. Vet. Med. Sci. 81(3), 473–479. Zhao, C., Zheng, H., Zhou, L., Ji, H., Zhao, L., Yu, W. and Gong, Q. 2021. Molecules falcarindiol isolated from notopterygium incisum inhibits the quorum sensing of Pseudomonas aeruginosa. Molecules 26(19), 5896. Available via https://doi.org/10.3390/molecules26195896 | ||
| How to Cite this Article |
| Pubmed Style Nwachukwu KC, Ugbogu OC, Nwosu IL, Nwarunma E. Anti-quorum sensing effects of medicinal plants and chemical compounds: A new dimension against Pseudomonas aeruginosa and Staphylococcus aureus. J Microbiol Infect Dis. 2023; 13(3): 98-109. doi:10.5455/JMID.2023.v13.i3.1 Web Style Nwachukwu KC, Ugbogu OC, Nwosu IL, Nwarunma E. Anti-quorum sensing effects of medicinal plants and chemical compounds: A new dimension against Pseudomonas aeruginosa and Staphylococcus aureus. https://www.jmidonline.org/?mno=302657408 [Access: January 25, 2026]. doi:10.5455/JMID.2023.v13.i3.1 AMA (American Medical Association) Style Nwachukwu KC, Ugbogu OC, Nwosu IL, Nwarunma E. Anti-quorum sensing effects of medicinal plants and chemical compounds: A new dimension against Pseudomonas aeruginosa and Staphylococcus aureus. J Microbiol Infect Dis. 2023; 13(3): 98-109. doi:10.5455/JMID.2023.v13.i3.1 Vancouver/ICMJE Style Nwachukwu KC, Ugbogu OC, Nwosu IL, Nwarunma E. Anti-quorum sensing effects of medicinal plants and chemical compounds: A new dimension against Pseudomonas aeruginosa and Staphylococcus aureus. J Microbiol Infect Dis. (2023), [cited January 25, 2026]; 13(3): 98-109. doi:10.5455/JMID.2023.v13.i3.1 Harvard Style Nwachukwu, K. C., Ugbogu, . O. C., Nwosu, . I. L. & Nwarunma, . E. (2023) Anti-quorum sensing effects of medicinal plants and chemical compounds: A new dimension against Pseudomonas aeruginosa and Staphylococcus aureus. J Microbiol Infect Dis, 13 (3), 98-109. doi:10.5455/JMID.2023.v13.i3.1 Turabian Style Nwachukwu, Kingsley Chukwuemeka, Ositadinma Chinyere Ugbogu, Ijeoma Linda Nwosu, and Ebubechukwu Nwarunma. 2023. Anti-quorum sensing effects of medicinal plants and chemical compounds: A new dimension against Pseudomonas aeruginosa and Staphylococcus aureus. Journal of Microbiology and Infectious Diseases, 13 (3), 98-109. doi:10.5455/JMID.2023.v13.i3.1 Chicago Style Nwachukwu, Kingsley Chukwuemeka, Ositadinma Chinyere Ugbogu, Ijeoma Linda Nwosu, and Ebubechukwu Nwarunma. "Anti-quorum sensing effects of medicinal plants and chemical compounds: A new dimension against Pseudomonas aeruginosa and Staphylococcus aureus." Journal of Microbiology and Infectious Diseases 13 (2023), 98-109. doi:10.5455/JMID.2023.v13.i3.1 MLA (The Modern Language Association) Style Nwachukwu, Kingsley Chukwuemeka, Ositadinma Chinyere Ugbogu, Ijeoma Linda Nwosu, and Ebubechukwu Nwarunma. "Anti-quorum sensing effects of medicinal plants and chemical compounds: A new dimension against Pseudomonas aeruginosa and Staphylococcus aureus." Journal of Microbiology and Infectious Diseases 13.3 (2023), 98-109. Print. doi:10.5455/JMID.2023.v13.i3.1 APA (American Psychological Association) Style Nwachukwu, K. C., Ugbogu, . O. C., Nwosu, . I. L. & Nwarunma, . E. (2023) Anti-quorum sensing effects of medicinal plants and chemical compounds: A new dimension against Pseudomonas aeruginosa and Staphylococcus aureus. Journal of Microbiology and Infectious Diseases, 13 (3), 98-109. doi:10.5455/JMID.2023.v13.i3.1 |