| Case Report | ||
J Microbiol Infect Dis. 2023; 13(4): 196-199 J. Microbiol. Infect. Dis., (2023), Vol. 13(4): 196–199 Case Report Tenofovir disoproxil fumarate-related toxicity: A case of Fanconi syndromeSameer Abdul Samad1*, Aparna Balachandran2, Rahul Nalayadam3 and Sajith Narayanan21Infectious Diseases, Aster MIMS, Kozhikode, India 2Department of Nephrology, Aster MIMS Kozhikode, Kozhikode, India 3Department of Internal Medicine, Aster MIMS Kozhikode, Kozhikode, India *Corresponding Author: Sameer Abdul Samad. Infectious Diseases, Aster MIMS, Kozhikode, India. Email: sameerinvogue [at] gmail.com Submitted: 28/09/2023 Accepted: 23/11/2023 Published: 31/12/2023 © 2023 Journal of Microbiology and Infectious Diseases
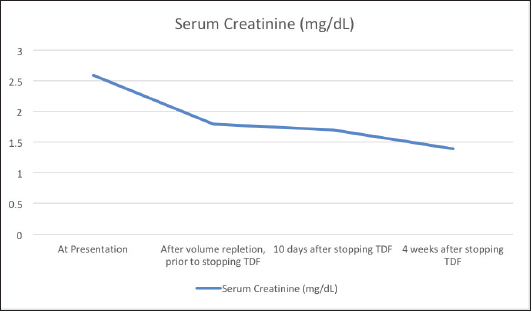
AbstractBackground: Tenofovir-based regimens remain the first choice for the treatment of human immunodeficiency virus infection and hepatitis B infection. Despite its virologic efficacy and ease of administration, it can lead to significant nephrotoxicity which may not be fully reversible. Case Description: An elderly gentleman on Tenofovir disoproxil fumarate (TDF)-based antiretroviral therapy for 10 years presented with vague symptoms of fatigue, poor oral intake and constipation. He was found to have hypokalemia and a volume depleted status. Detailed evaluation revealed hyperchloremic metabolic acidosis, hypophosphatemia, hypouricemia, proteinuria and amino aciduria consistent with a diagnosis of Fanconi syndrome. He was switched to Abacavir-based regimen, and supplements to correct acidosis and electrolytes were given. At the follow-up visit after a month, his proximal renal tubular injury recovered partially and he was symptomatically better. Conclusion: Patients on long-term therapy with tenofovir should be monitored for proximal renal tubular injury besides estimation of the glomerular filtration rate. Fanconi syndrome may present late after several years of being on TDF therapy. Partial or complete recovery of the renal tubular function occurs after stopping tenofovir. Keywords: Tenofovir, TDF, Fanconi, Proximal tubule, Hypophosphatemia. IntroductionTenofovir disoproxil fumarate (TDF) is recommended as a component of the first-line antiretroviral treatment regimens in treatment-naïve, Human immunodeficiency virus (HIV) infected patients due to its high virologic efficacy and the ease of once daily dosing (NACO, 2021). Despite its good safety profile in the initial periods of treatment, its long-term use is significantly associated with renal damage (Fernandez-Fernandez et al., 2011). Tenofovir is excreted from the body by a combination of glomerular filtration and proximal tubular secretion. In individuals who are genetically predisposed due to polymorphisms of the renal transporter molecules, tenofovir accumulates in the proximal renal tubular cells and causes mitochondrial damage. This can lead to Fanconi syndrome characterized by the loss of substances that are normally reabsorbed at the proximal nephron—bicarbonate, amino acids, glucose, phosphate, and protein (Moss et al., 2014). Tenofovir is believed to be non-toxic to the glomeruli, and modest elevations in serum creatinine is usually reversible with the cessation of the drug. But tubular damage leading to Fanconi syndrome which occurs in less than 0.1% of individuals taking tenofovir may not be totally reversible (Nelson et al., 2007). Therefore, careful evaluation and diagnosis are needed for patients on tenofovir therapy to adopt an appropriate management plan. Case DetailsA 70-year-old male who was diagnosed with HIV infection and had been on anti-retroviral therapy with a combination of TDF, Emtricitabine, and Efavirenz for 10 years presented with a history of loss of appetite, severe fatigue, and poor oral intake since 2 weeks. There was also a history of mild intermittent pain over the back and ribs. There was a history of laxative abuse which he took for constipation. On examination his blood pressure was normal, speech was slurred, there was a mild decrease in the power of bilateral upper and lower limbs, and fissured tongue and glossitis were present. The patient was found to have a volume-depleted status with hypokalemia (serum potassium 1.8 mEq/l), hypernatremia (147 mEq/l), and raised serum creatinine (2.6 mg/dl). Adequate hydration was given and on further evaluation he was found to have hyperchloremic (serum chloride 120 mEq/l) metabolic acidosis with an anion gap of 12.6 mEq/l, hypophosphatemia (1.8 mg/dl) with fractional urinary excretion of phosphate >50%, hypouricemia (1.5 mg/dl), renal glycosuria (brick red appearance on Benedict’s test of urine, random blood sugar of 138 mg/dl), albuminuria (368 mg/g of creatinine), urine anion gap of 25 and urine pH of 5.5 which increased to 7.0 following bicarbonate supplementation. Amino aciduria was also present, the following amino acids were detected in urine—alanine, citrulline, glycine, methionine, and phenyl alanine. Ultrasound of the abdomen showed normal-sized kidneys with bilateral raised renal cortical echotexture and cortical cysts, the largest of them measuring 3.1 × 3.1 cm in the left kidney. His blood sugar levels remained in the normal range during the hospital stay. Thyroid stimulating hormone level was 1.63 mIU/l. The serum parathormone level was 57.9 pg/ml which ruled out hyperparathyroidism as a cause of hypophosphatemia. His quantitative HIV ribonucleic acid polymerase chain reaction did not detect any viral copies, and his CD4 T-lymphocyte count was 651/µl. Workup for opportunistic infections was unrevealing except for esophagitis with Cytomegalovirus (CMV) detected in the esophageal tissue biopsy with absent CMV viremia. Symptoms of esophagitis resolved after a 3 week’s course of valganciclovir. Dual energy X-ray absorptiometry showed osteopenia and his serum calcium and 25-hydroxy cholecalciferol levels were normal. TDF was stopped and he was switched to Abacavir-based anti-retroviral regimen (Abacavir, Lamivudine, and Dolutegravir). Bicarbonate, phosphate, and potassium supplementation were added. At a month’s follow-up, he was symptomatically quite better, his serum creatinine levels decreased and proteinuria improved (Fig. 1). Phosphate supplementation could be stopped in the 10th week following discontinuation of TDF after its serum levels normalized. DiscussionThe vague symptoms of fatigue, loss of appetite, constipation, and intermittent pain over the bones in our patient were later identified to be due to renal wastage of potassium, phosphorous, and protein and metabolic acidosis. Fanconi syndrome results from the breakdown of solute transport in the proximal tubule and is characterized by hypophosphatemia, hypouricemia, normoglycemic glycosuria, metabolic acidosis, low-molecular-weight proteinuria, and aminoaciduria. The estimated glomerular filtration rate and urine albumin/creatinine ratio, which are frequently used to monitor acute kidney injury and chronic kidney disease, are not sensitive markers of proximal tubular function. As noted in the case described above, urinary phosphate wasting can also lead to bone demineralization (Hall et al., 2014). In children, Fanconi syndrome can occur due to genetic conditions like cystinosis, galactosemia, or hereditary fructose intolerance. In adults, the most important causes of acquired Fanconi syndrome are drugs and heavy metals (Keefe and Bokhari, 2023). SLC22A6, SLC22A7, and SLC22A8 (organic anion transporter 1, 2, and 3, respectively) are transporters present on the basolateral membrane of the proximal renal tubular cells and function to transport tenofovir into the cells (Uwai et al., 2007). ABCC4 is the main efflux transporter of tenofovir across the apical surface of the proximal tubular cells (Kohler et al., 2011). Cellular accumulation of tenofovir due to dysfunction of the transporter molecules leads to inhibition of mitochondrial DNA polymerase-γ and proximal tubular dysfunction (Lewis, 2003; Perazella, 2010).
Fig. 1. Trend of creatinine levels in the serum before and after stopping Tenofovir disoproxil fumarate. Fanconi syndrome due to TDF was first reported in the literature in 2002 (Verhelst et al., 2002). Further reports have followed, but it remains a rare complication of TDF therapy occurring in less than 0.1% of TDF-treated HIV patients (Nelson et al., 2007). Risk factors for renal tubular dysfunction include older age, reduced body mass, pre-existing renal impairment and simultaneous use of other nephrotoxic medications and onset is frequently after several months of TDF-based therapy (Rodriguez-Nvoa et al., 2010; Nishijima et al., 2011). Other predisposing factors that lead to more severe forms of TDF-related renal toxicity include use of concomitant protease inhibitors and genetic polymorphisms of renal transporters of tenofovir (Izzedine et al., 2006; Gupta, 2008; Pushpakom et al., 2011). The mainstay of therapy is withdrawal of the offending drug, which results in regeneration of the proximal tubule, although this can take months and residual defects may remain (Gupta et al., 2014). Alternative therapies include other nucleoside reverse transcriptase inhibitors such as Abacavir, and tenofovir alafenamide which is not a substrate of the SLC22A6 or SLC22A8 transporters and thus concentrates less in the tubular cells (Bam et al., 2014; Post et al., 2017). Tenofovir alafenamide is a prodrug that remains stable in plasma and permits administration of a much lower dose due to its ability to target the infected CD4 T-lymphocytes by selective intracellular hydrolysis and thus has a better renal safety profile (Ray et al., 2016). ConclusionPatients can present with Fanconi syndrome even after a decade of being on TDF therapy. They may present with vague symptoms, and careful investigations should be undertaken to monitor and evaluate patients on long-term tenofovir therapy. ReferencesBam, R.A., Yant, S.R., Cihlar, T. 2014. Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir. Ther. 19(7), 687–692. Fernandez-Fernandez, B., Montoya-Ferrer, A., Sanz, A.B., Sanchez-Niño, M.D., Izquierdo, M.C., Poveda, J., Sainz-Prestel, V., Ortiz-Martin, N., Parra-Rodriguez, A., Selgas, R., Ruiz-Ortega, M., Egido, J. and Ortiz, A. (2011). Tenofovir nephrotoxicity: 2011 update. AIDS. Res. Treat. 2011, 1–11. Gupta, S.K. (2008). Tenofovir-associated fanconi syndrome: review of the FDA adverse event reporting system. AIDS. Patient. Care. STDS. 22(2), 99–103. Available via Https://Home.Liebertpub.Com/Apc Gupta, S.K., Anderson, A.M., Ebrahimi, R., Fralich, T., Graham, H., Scharen-Guivel, V., Flaherty, J.F., Fortin, C., Kalayjian, R.C., Rachlis, A. and Wyatt, C.M. (2014). Fanconi syndrome accompanied by renal function decline with tenofovir disoproxil fumarate: a prospective, case-control study of predictors and resolution in HIV-infected patients. PloS One 9(3), e92717. Hall, A.M., Bass, P. and Unwin, R.J. (2014). Drug-induced renal fanconi syndrome. QJM. 107(4), 261–269. Izzedine, H., Hulot, J.S., Villard, E., Goyenvalle, C., Dominguez, S., Ghosn, J., Valentin, M.A., Lechat, P. and Deray, G. (2006). Association between ABCC2 gene haplotypes and tenofovir-induced proximal tubulopathy. J. Infect. Dis. 194(11), 1481–1491. Keefe, P. and Bokhari, S.R.A. (2023). Fanconi syndrome. St. Petersburg, FL: StatPearls. Available via https://www.ncbi.nlm.nih.gov/books/NBK534872/ Kohler, J.J., Hosseini, S.H., Green, E., Abuin, A., Ludaway, T., Russ, R., Santoianni, R. and Lewis, W. (2011). Tenofovir renal proximal tubular toxicity is regulated by OAT1 and MRP4 transporters. Lab. Invest. J. Tech. Methods. Pathol. 91(6), 852. Lewis, W. (2003). Mitochondrial dysfunction and nucleoside reverse transcriptase inhibitor therapy: experimental clarifications and persistent clinical questions. Antivir. Res. 58(3), 189–197. Moss, D.M., Neary, M. and Owen, A. (2014). The role of drug transporters in the kidney: lessons from tenofovir. Front. Pharmacol. 5(NOV), 118145. Nelson, M.R., Katlama, C., Montaner, J.S., Cooper, D.A., Gazzard, B., Clotet, B., Lazzarin, A., Schewe, K., Lange, J., Wyatt, C., Curtis, S., Chen, S.S., Smith, S., Bischofberger, N. and Rooney, J.F. (2007). The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS 21(10), 1273–1281. Nishijima, T., Komatsu, H., Gatanaga, H., Aoki, T., Watanabe, K., Kinai, E., Honda, H., Tanuma, J., Yazaki, H., Tsukada, K., Honda, M., Teruya, K., Kikuchi, Y. and Oka, S. (2011). Impact of small body weight on tenofovir-associated renal dysfunction in HIV-infected patients: a retrospective cohort study of Japanese patients. PloS One 6(7), e22661. NACO. (2021). Care, support &treatment National AIDS Control Organization MoHFW Government of India. NACO. Available via https://naco.gov.in/care-support-treatment (Accessed 26 September 2023) Perazella, M.A. (2010). Tenofovir-induced kidney disease: an acquired renal tubular mitochondriopathy. Kidney. Int. 78(11), 1060–1063. Post, F.A., Tebas, P., Clarke, A., Cotte, L., Short, W.R., Abram, M.E., Jiang, S., Cheng, A., Das, M. and Fordyce, M.W. (2017). Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected adults with renal impairment: 96-week results from a single-arm, multicenter, open-label phase 3 study. J. Acquir. Immune. Defic. Syndr. 74(2), 180–184. Pushpakom, S.P., Liptrott, N.J., Rodríguez-Nóvoa, S., Labarga, P., Soriano, V., Albalater, M., Hopper-Borge, E., Bonora, S., Di Perri, G., Back, D.J., Khoo, S., Pirmohamed, M. and Owen, A. (2011). Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J. Infect. Dis. 204(1), 145–153. Ray, A.S., Fordyce, M.W. and Hitchcock, M.J.M. (2016). Tenofovir alafenamide: a novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus. Antivir. Res. 125, 63–70. Rodriguez-Nvoa, S., Alvarez, E., Labarga, P. and Soriano, V. (2010). Renal toxicity associated with tenofovir use. Expert. Opin. Drug. Saf. 9(4), 545–559. Uwai, Y., Ida, H., Tsuji, Y., Katsura, T. and Inui, K.I. (2007). Renal transport of adefovir, cidofovir, and tenofovir by SLC22A family members (hOAT1, hOAT3, and hOCT2). Pharm. Res. 24(4), 811–815. Verhelst, D., Monge, M., Meynard, J.L., Fouqueray, B., Mougenot, B., Girard, P.M., Ronco, P. and Rossert, J. (2002). Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am. J. Kidney. Dis. Off. J. Nat. Kidney. Found. 40(6), 1331–1333. | ||
| How to Cite this Article |
| Pubmed Style Samad SA, Balachandran A, Nalayadam R, Narayanan S. Tenofovir disoproxil fumarate-related toxicity: A case of Fanconi syndrome. J Microbiol Infect Dis. 2023; 13(4): 196-199. doi:10.5455/JMID.2023.v13.i4.5 Web Style Samad SA, Balachandran A, Nalayadam R, Narayanan S. Tenofovir disoproxil fumarate-related toxicity: A case of Fanconi syndrome. https://www.jmidonline.org/?mno=171158 [Access: January 25, 2026]. doi:10.5455/JMID.2023.v13.i4.5 AMA (American Medical Association) Style Samad SA, Balachandran A, Nalayadam R, Narayanan S. Tenofovir disoproxil fumarate-related toxicity: A case of Fanconi syndrome. J Microbiol Infect Dis. 2023; 13(4): 196-199. doi:10.5455/JMID.2023.v13.i4.5 Vancouver/ICMJE Style Samad SA, Balachandran A, Nalayadam R, Narayanan S. Tenofovir disoproxil fumarate-related toxicity: A case of Fanconi syndrome. J Microbiol Infect Dis. (2023), [cited January 25, 2026]; 13(4): 196-199. doi:10.5455/JMID.2023.v13.i4.5 Harvard Style Samad, S. A., Balachandran, . A., Nalayadam, . R. & Narayanan, . S. (2023) Tenofovir disoproxil fumarate-related toxicity: A case of Fanconi syndrome. J Microbiol Infect Dis, 13 (4), 196-199. doi:10.5455/JMID.2023.v13.i4.5 Turabian Style Samad, Sameer Abdul, Aparna Balachandran, Rahul Nalayadam, and Sajith Narayanan. 2023. Tenofovir disoproxil fumarate-related toxicity: A case of Fanconi syndrome. Journal of Microbiology and Infectious Diseases, 13 (4), 196-199. doi:10.5455/JMID.2023.v13.i4.5 Chicago Style Samad, Sameer Abdul, Aparna Balachandran, Rahul Nalayadam, and Sajith Narayanan. "Tenofovir disoproxil fumarate-related toxicity: A case of Fanconi syndrome." Journal of Microbiology and Infectious Diseases 13 (2023), 196-199. doi:10.5455/JMID.2023.v13.i4.5 MLA (The Modern Language Association) Style Samad, Sameer Abdul, Aparna Balachandran, Rahul Nalayadam, and Sajith Narayanan. "Tenofovir disoproxil fumarate-related toxicity: A case of Fanconi syndrome." Journal of Microbiology and Infectious Diseases 13.4 (2023), 196-199. Print. doi:10.5455/JMID.2023.v13.i4.5 APA (American Psychological Association) Style Samad, S. A., Balachandran, . A., Nalayadam, . R. & Narayanan, . S. (2023) Tenofovir disoproxil fumarate-related toxicity: A case of Fanconi syndrome. Journal of Microbiology and Infectious Diseases, 13 (4), 196-199. doi:10.5455/JMID.2023.v13.i4.5 |