| Case Report | ||
J. Microbiol. Infect. Dis., (2023), Vol. 13(2): 93–97 Case Report Medical management of Pseudomonas aeruginosa endocarditis: A case reportSameer Abdul Samad1*, Priya T. Nair2, Abeed Hussain2, Amal Byju2, Shafeeq Mattummal3 and Ravi Arodiyil41Department of Infectious Diseases, Aster Malabar Institute of Medical Sciences, Calicut, India 2Department of Internal Medicine, Aster Malabar Institute of Medical Sciences, Calicut, India 3Department of Cardiology, Aster Malabar Institute of Medical Sciences, Calicut, India 4Department of Otorhinolaryngology, Aster Malabar Institute of Medical Sciences, Calicut, India *Corresponding Author: Sameer Abdul Samad. Department of Infectious Diseases, Aster MIMS Hospital, Calicut, India. Email: sameerinvogue [at] gmail.com Submitted: 11/05/2023 Accepted: 11/06/2023 Published: 30/06/2023 © 2023 Journal of Microbiology and Infectious Diseases
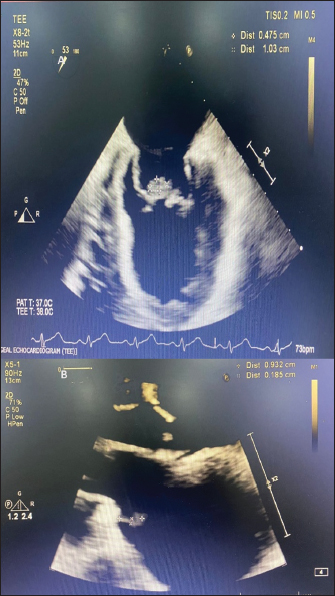
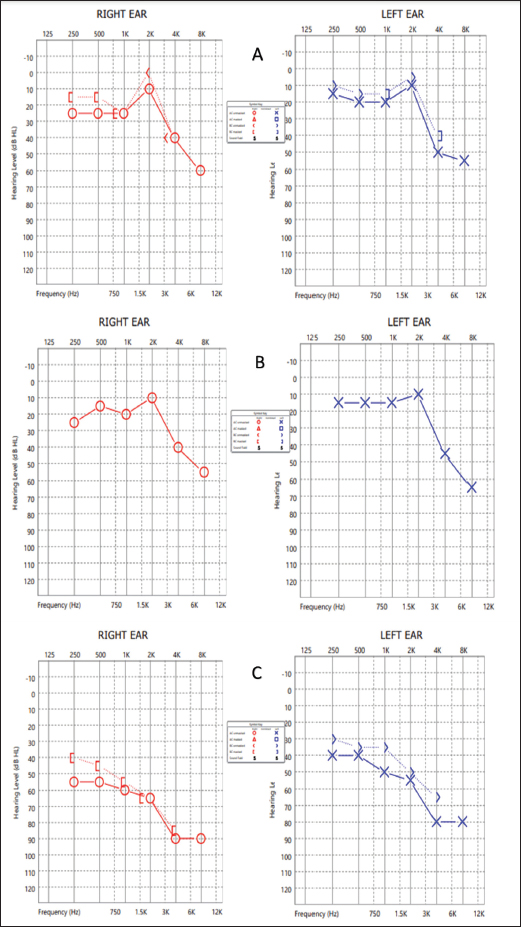
AbstractBackground: Pseudomonas aeruginosa is a rare cause of infective endocarditis. There is no standard treatment regimen for the management of infective endocarditis caused by this organism. Pseudomonas aeruginosa produces biofilm on the endocardial surface which can be difficult to eradicate, and monotherapy with a traditional beta-lactam agent may fail due to inadequate penetration into biofilm and low or absent activity on non-dividing cells. Prolonged courses and surgical intervention may be required to treat this infection. Case Description: A case of infective endocarditis caused by P. aeruginosa which showed inadequate response to beta-lactam plus beta-lactamase-inhibitor therapy and was later successfully managed without surgical intervention but a combination of a beta-lactam and an aminoglycoside is described here. This case which was treated with amikacin was followed up for ototoxicity. Ototoxicity is an irreversible side effect of amikacin and close follow-up with serial audiograms is required during therapy, especially when therapeutic drug monitoring is not possible. Conclusion: A combination regimen of a beta-lactam active against P. aeruginosa plus an aminoglycoside for a duration of four weeks can be an effective treatment for infective endocarditis caused by P. aeruginosa. Periodic monitoring for adverse drug events should be undertaken during therapy. Keywords: Amikacin, Endocarditis, Haemodialysis, Mitral, Ototoxicity. IntroductionInfective endocarditis due to non-HACEK (not belonging to any of the following genera: Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, and Kingella) Gram-negative bacilli is rare, and that is due to Pseudomonas aeruginosa constitutes about 0.4% of all cases (Morpeth et al., 2007). Because of the rarity of such cases, there are no strong treatment recommendations. Here, we describe a case of P. aeruginosa endocarditis in a patient with multiple comorbidities, who was receiving maintenance hemodialysis through a functioning arterio-venous fistula. Treatment failed with beta-lactam plus beta-lactamase-inhibitor therapy and the patient was subsequently managed with a combination of a beta-lactam with an aminoglycoside showing prompt clinical and radiological resolution. Case DetailsA 57-year-old male patient known to have diabetes mellitus, hypertension, chronic liver disease with portal hypertension, coronary artery disease, and chronic kidney disease receiving maintenance hemodialysis thrice a week for the past 4 years presented to our hospital with low-grade intermittent fever of 3 weeks duration. There were no localizing symptoms. General examination revealed a temperature of 38.8°C, pallor, mild icterus, and splinter hemorrhages on the nails of the left middle and ring fingers. Systemic examination revealed splenomegaly and grade 3 pan-systolic murmur in the mitral area. Laboratory investigations showed elevated erythrocyte sedimentation rate and serum C-reactive protein, mild anemia, and thrombocytopenia. After sending a set of blood cultures, he was started empirically on intravenous (IV) piperacillin-tazobactam 2.25 g thrice a day and Vancomycin 500 mg IV once daily each dose infused over 30 minutes. Computed tomography of the thorax revealed prominent mediastinal nodes, the largest measuring 1.2 cm on the short axis. Trans-thoracic echocardiography was done which showed probable vegetation measuring 19 × 3 mm on the anterior mitral leaflet (AML) and posterior mitral leaflet, and severe mitral regurgitation. A trans-esophageal echocardiography was done and confirmed the findings (Fig. 1A). Bronchoscopy and endobronchial ultrasound-guided fine needle aspiration of the enlarged mediastinal lymph node were done and were unrevealing. Two sets of blood cultures which were sent 4 days apart both grew P. aeruginosa sensitive to piperacillin-tazobactam [Minimum inhibitory concentration (MIC) – 8 µg/ml], amikacin (MIC - 4 µg/ml), ceftazidime (MIC - 2 µg/ml) and cefepime (MIC - 2 µg/ml). A previous blood culture which was sent from a local hospital prior to admission at our centre was also traced and had grown P. aeruginosa with a similar susceptibility pattern. IV piperacillin-tazobactam in a renally adjusted dose was continued and vancomycin was stopped based on susceptibility testing results. The need for valve replacement due to the difficulty in eradicating the infection with medical therapy alone was explained to the patient’s relatives, however, he was unfit for surgery at that point of time. Even after 2 weeks of IV piperacillin-tazobactam 2.25 g given three times a day, fever spikes persisted. We changed the regimen to a combination of a beta-lactam and an aminoglycoside - IV Ceftazidime 1 g once daily and IV Amikacin 750 mg on alternate days. He underwent pure tone audiometry (PTA) screening prior to initiation of IV amikacin therapy and it was suggestive of bilateral minimal sensory neural hearing loss - 25 decibels (db) in right ear and left ear each (Fig. 2A). Considering the risks and benefits, it was decided to continue this therapy and monitor with periodic audiograms. Fever spikes decreased over the next 3 days, his inflammatory markers reduced and he got symptomatically better. He was discharged from hospital with advice to continue IV antibiotics from home. He became completely afebrile after a week of starting the combination antibiotic regimen. Echocardiography repeated after a week at a follow-up visit showed decreased size of the vegetation on AML to 3 × 8 mm (Fig. 1B). There was no worsening of hearing as assessed by PTA repeated after a week of starting amikacin (Fig. 2B). An echocardiography repeated after 3 weeks showed complete resolution of the vegetation on AML and decrease in the size of vegetation on PML to a tiny strand-like structure. Meanwhile he also complained of hearing loss. PTA was repeated and showed moderately severe bilateral sensory neural hearing loss - 68 db in the right ear and 56 db in the left ear (Fig. 2). Hence the antibiotic regimen was stopped and a short course of prednisolone 1 mg/kg tapered over 2 weeks was given without much symptomatic benefit. He was later planned for cochlear implantation as a hearing aid. DiscussionNon-HACEK Gram-negative endocarditis is often associated with health-care contact. Among the Non-HACEK Gram-negative bacilli, Escherichia coli has been found to be the most common cause of infective endocarditis, followed by P. aeruginosa (Morpeth et al., 2007; Falcone et al., 2018). Pseudomonas aeruginosa endocarditis was once thought to be primarily a disease of injection drug users (Wieland et al., 1986; Morpeth et al., 2007). But later reviews have found that the majority of Pseudomonas endocarditis cases occurred among patients with healthcare contact rather than IV drug users (Morpeth et al., 2007; Lin et al., 2016). Our patient had frequent contact with healthcare facility during the thrice-weekly hemodialysis sessions and the likely portal of entry for the pathogen was the IV access.
Fig. 1. (A): Transesophageal echocardiography showing vegetation on anterior and posterior mitral leaflets prior to starting combination therapy. (B): Trans-thoracic echocardiography done after a week of combination antibiotic therapy showing decrease in size of vegetation on AML.
Fig. 2. PTA of both ears at (A): baseline prior to start of amikacin therapy, (B): after a week of starting IV amikacin and (C): 4th week of amikacin. Biofilms are formed on the endocardial surface in infective endocarditis (Elgharably et al., 2016). Pseudomonas aeruginosa is known to produce biofilms that enhance its pathogenicity, and protect it from the immune system. Pseudomonas aeruginosa can adopt either the sessile or the planktonic form during growth. On natural surfaces the planktonic form aggregates and produces an adhesive matrix, forming a biofilm which is an adaptive response to growth in an unsuitable environment. The biofilms are 10–1,000 times more resistant to antibiotics than planktonic cells due to the poor penetration of antibiotics into the matrix as well as altered metabolic activity and protein synthesis of the organisms (Tuon et al., 2022). Thus, traditional beta-lactams may not be sufficient to eradicate P. aeruginosa from biofilms. Historically, adjunctive aminoglycosides which act on the 30S ribosome have been used in the treatment of endocarditis due to Enterococci, Streptococci, and Staphylococci because of their in-vivo synergism with beta-lactams which are cell wall active agents, and their ability to reduce tolerance to penicillin (Jawetz et al., 1950). In the treatment of P. aeruginosa infections also, a combination of an aminoglycoside with a beta-lactam is used owing to the synergistic action when the organism is susceptible to both agents and effective bacterial killing when the beta-lactam agent is resistant (Avent et al., 2022). This is particularly useful in the treatment of infective endocarditis considering the long duration of antibiotic therapy required and the propensity of Pseudomonas to acquire resistance during treatment. Meropenem exposure is associated with the highest risk of resistance development during treatment in P. aeruginosa (Ong et al., 2011). However, the use of aminoglycosides carries a significant risk of nephrotoxicity and ototoxicity. Our patient described above had end-stage renal disease and was receiving hemodialysis thrice a week. The dose of amikacin was timed accordingly to be given after each dialysis session (Heintz et al., 2009). There was no facility available to do therapeutic drug monitoring of amikacin. After explaining the situation to the patient’s relatives regarding the need for aggressive medical management and the possible adverse effects, we continued as per the available recommendations, and serial audiograms were done to monitor hearing. Prolonged courses of amikacin have been used in the treatment of tuberculosis. It is one of the group C agents in the World Health Organization guidelines for the treatment of multi-drug resistant tuberculosis, and it can be given up to 6 months as part of a shortened course of multi-drug therapy. The risk of ototoxicity increases steeply after 6 months of therapy, can progress even after cessation of the drug, and is usually irreversible (Duggal and Sarkar, 2007). Therapeutic drug monitoring should be undertaken during amikacin therapy if such a facility is available. The cumulative amikacin exposure as determined by the cumulative area under the concentration-time curve and duration of therapy, rather than peak and trough plasma levels, are predictors of ototoxicity (Duggal and Sarkar, 2007; Modongo et al., 2015). ConclusionCareful evaluation is required to confirm a diagnosis of infective endocarditis caused by P. aeruginosa, including multiple sets of blood cultures and trans-esophageal echocardiography. A combination regimen with synergistic activity for a duration of 4 weeks can be an effective treatment without surgical intervention. Therapeutic drug monitoring of aminoglycosides should be done where the facility is available. AcknowledgmentsWe thank all the doctors and staff involved in caring for the patient. Conflict of interestThe authors have declared that no competing interests exist. FundingThe authors have no funding to report. ReferencesAvent, M.L., McCarthy, K.L., Sime, F.B., Naicker, S., Heffernan, A.J., Wallis, S.C., Paterson, D.L. and Roberts, J.A. 2022. Evaluating mono- and combination therapy of meropenem and amikacin against Pseudomonas aeruginosa Bacteremia in the Hollow-Fiber infection model. Microbiol. Spectr. 10(3), e0052522; doi: 10.1128/spectrum.00525-22. Duggal, P. and Sarkar, M. 2007. Audiologic monitoring of multi-drug resistant tuberculosis patients on aminoglycoside treatment with long term follow-up. BMC. Ear. Nose. Throat. Disord. 7, 5; doi: 10.1186/1472-6815-7-5. Elgharably, H., Hussain, S.T., Shrestha, N.K., Blackstone, E.H. and Pettersson, G.B. 2016. Current hypotheses in cardiac surgery: biofilm in infective endocarditis. Semin. Thorac. Cardiovasc. Surg. 28(1), 56–59; doi: 10.1053/j.semtcvs.2015.12.005. Falcone, M., Tiseo, G., Durante-Mangoni, E., Ravasio, V., Barbaro, F., Ursi, M.P., Pasticci, M.B., Bassetti, M., Grossi, P., Venditti, M. and Rizzi, M. 2018. Risk factors and outcomes of endocarditis due to non-HACEK Gram-negative Bacilli: data from the prospective multicenter Italian endocarditis study cohort. Antimicrob. Agents. Chemother. 62(4), e02208–e02217; doi: 10.1128/AAC.02208-17. Heintz, B.H., Matzke, G.R. and Dager, W.E. 2009. Antimicrobial dosing concepts and recommendations for critically ill adult patients receiving continuous renal replacement therapy or intermittent hemodialysis. Pharmacotherapy 29(5), 562–577; doi: 10.1592/phco.29.5.562. Jawetz, E., Gunnison, J.B. and Coleman, V.R. 1950. The combined action of penicillin with streptomycin or chloromycetin on Enterococci in vitro. Science 111(2880), 254–256; doi: 10.1126/science.111.2880.254. Lin, T.I., Huang, Y.F., Liu, P.Y., Chou, C.A., Chen, Y.S., Chen, Y.Y., Hsieh, K.S. and Chen, Y.S. 2016. Pseudomonas aeruginosa infective endocarditis in patients who do not use intravenous drugs: analysis of risk factors and treatment outcomes. J. Microbiol. Immunol. Infect. 49(4), 516–522; doi: 10.1016/j.jmii.2014.08.019. Modongo, C., Pasipanodya, J.G., Zetola, N.M., Williams, S.M., Sirugo, G. and Gumbo, T. 2015. Amikacin concentrations predictive of ototoxicity in multidrug-resistant tuberculosis patients. Antimicrob. Agents. Chemother. 59(10), 6337–6343; doi: 10.1128/AAC.01050-15. Morpeth, S., Murdoch, D., Cabell, C.H., Karchmer, A.W., Pappas, P., Levine, D., Nacinovich, F., Tattevin, P., Fernández-Hidalgo, N., Dickerman, S., Bouza, E., del Río, A., Lejko-Zupanc, T., de Oliveira Ramos, A., Iarussi, D., Klein, J., Chirouze, C., Bedimo, R., Corey, G.R., Fowler, V.G. and International collaboration on endocarditis prospective cohort study (ICE-PCS) investigators. 2007. Non-HACEK gram-negative Bacillus endocarditis. Ann. Intern. Med. 147(12), 829–835; doi: 10.7326/0003-4819-147-12-200712180-00002. Ong, D.S., Jongerden, I.P., Buiting, A.G., Leverstein-van, H., Maurine, A., Speelberg, B., Kesecioglu, J. and Bonten, M.J.M. 2011. Antibiotic exposure and resistance development in Pseudomonas aeruginosa and Enterobacter species in intensive care units. Crit. Care. Med. 39(11), 2458–2463; doi: 10.1097/CCM.0b013e318225756d. Tuon, F.F., Dantas, L.R., Suss, P.H. and Ribeiro, V.S.T. 2022. Pathogenesis of the Pseudomonas aeruginosa Biofilm: a review. Pathogens 11(3), 300; doi: 10.3390/pathogens11030300. Wieland, M., Lederman, M.M., Kline-King, C., Keys, T.F., Lerner, P.I., Steven, N., Chemilewski, R., Banks, V.D. and Ellner, J.J. 1986. Left-sided endocarditis due to Pseudomonas aeruginosa. A report of 10 cases and review of the literature. Medicine (Baltimore) 65(3), 180–189; doi: 10.1097/00005792-198605000-00006. | ||
| How to Cite this Article |
| Pubmed Style Samad SA, Nair PT, Hussain A, Byju A, Mattummal S, Arodiyil R. Medical management of Pseudomonas aeruginosa endocarditis: A case report. J Microbiol Infect Dis. 2023; 13(2): 93-97. doi:10.5455/JMID.2023.v13.i2.8 Web Style Samad SA, Nair PT, Hussain A, Byju A, Mattummal S, Arodiyil R. Medical management of Pseudomonas aeruginosa endocarditis: A case report. https://www.jmidonline.org/?mno=153010 [Access: January 25, 2026]. doi:10.5455/JMID.2023.v13.i2.8 AMA (American Medical Association) Style Samad SA, Nair PT, Hussain A, Byju A, Mattummal S, Arodiyil R. Medical management of Pseudomonas aeruginosa endocarditis: A case report. J Microbiol Infect Dis. 2023; 13(2): 93-97. doi:10.5455/JMID.2023.v13.i2.8 Vancouver/ICMJE Style Samad SA, Nair PT, Hussain A, Byju A, Mattummal S, Arodiyil R. Medical management of Pseudomonas aeruginosa endocarditis: A case report. J Microbiol Infect Dis. (2023), [cited January 25, 2026]; 13(2): 93-97. doi:10.5455/JMID.2023.v13.i2.8 Harvard Style Samad, S. A., Nair, . P. T., Hussain, . A., Byju, . A., Mattummal, . S. & Arodiyil, . R. (2023) Medical management of Pseudomonas aeruginosa endocarditis: A case report. J Microbiol Infect Dis, 13 (2), 93-97. doi:10.5455/JMID.2023.v13.i2.8 Turabian Style Samad, Sameer Abdul, Priya T. Nair, Abeed Hussain, Amal Byju, Shafeeq Mattummal, and Ravi Arodiyil. 2023. Medical management of Pseudomonas aeruginosa endocarditis: A case report. Journal of Microbiology and Infectious Diseases, 13 (2), 93-97. doi:10.5455/JMID.2023.v13.i2.8 Chicago Style Samad, Sameer Abdul, Priya T. Nair, Abeed Hussain, Amal Byju, Shafeeq Mattummal, and Ravi Arodiyil. "Medical management of Pseudomonas aeruginosa endocarditis: A case report." Journal of Microbiology and Infectious Diseases 13 (2023), 93-97. doi:10.5455/JMID.2023.v13.i2.8 MLA (The Modern Language Association) Style Samad, Sameer Abdul, Priya T. Nair, Abeed Hussain, Amal Byju, Shafeeq Mattummal, and Ravi Arodiyil. "Medical management of Pseudomonas aeruginosa endocarditis: A case report." Journal of Microbiology and Infectious Diseases 13.2 (2023), 93-97. Print. doi:10.5455/JMID.2023.v13.i2.8 APA (American Psychological Association) Style Samad, S. A., Nair, . P. T., Hussain, . A., Byju, . A., Mattummal, . S. & Arodiyil, . R. (2023) Medical management of Pseudomonas aeruginosa endocarditis: A case report. Journal of Microbiology and Infectious Diseases, 13 (2), 93-97. doi:10.5455/JMID.2023.v13.i2.8 |