| Research Article | ||
J. Microbiol. Infect. Dis., (2024), Vol. 15(1): 19–31 Research Article Utility of PCR for the molecular characterization of virulence genes in bacteria associated with urinary tract infections in diabetic patientsBetty Daniel1, Mehvish Saleem1* and Syed Mudasir Andrabi21Department of Botany, St. Josephs College, Postgraduate and Research Centre, Bangalore, India 2Division of Animal Biotechnology, Shere Kashmir University of Agriculture and Technology (SKAUST –K), Srinagar, India *Corresponding Author: Mehvish Saleem. Department of Botany, St. Josephs College, Postgraduate and Research Centre, Bangalore, India. Email: mehvish.saleem [at] gmail.com Submitted: 12/9/2024 Accepted: 15/01/2025, Published: 31/03/2025 © 2025 Journal of Microbiology and Infectious Diseases
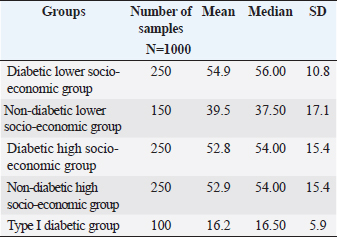
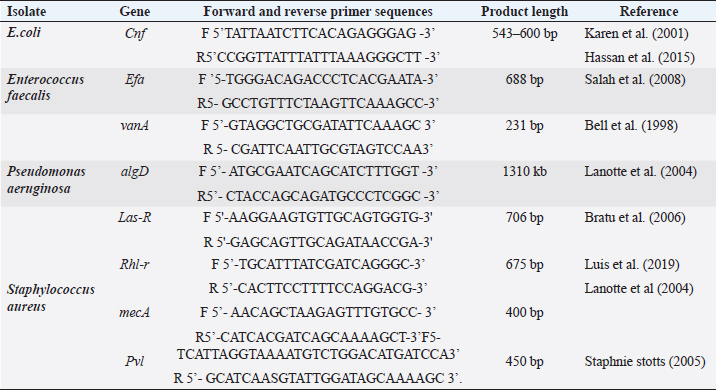
ABSTRACTBackground: Urinary tract infection (UTI) is a significant health issue, ranked as the second most common infection more, prevalent in patients with diabetes mellitus (DM). UTI is often associated with severe complications, such as cystitis, pyelonephritis, and renal or perinephric abscesses, especially in patients with poor glycemic control. Aim: The aim of this study was to identify the potential virulence genes (cnf1, vanA, efa, AlgD, las-r, rhl-R, mecA and pvl,) present in bacteria causing UTI thus contributing to the progression of infection among diabetic patients. Methods: One Thousand diabetic and non-diabetic urine samples were collected from various hospitals and diabetic centers across Bangalore city. Clean voided midstream urine samples were collected in sterile containers. Urine samples were processed following standard laboratory protocol. Results: Commonly recovered UTI isolates were Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, and Staphylococcus aureus. Antibiotic susceptibility was performed using the disc diffusion method (Kirby-Beur). The results of antibiotic sensitivity tests were recorded by measuring the Zone of inhibition. We used plasmid DNA isolation and polymerase chain reaction (PCR) for the detection of six different genes among the five predominant pathogens responsible for UTI. The cnf1 gene, encoding cytotoxic necrotizing factor, was detected in seven E. coli isolates n=15 (46%), with a gene size of 600 bp. The VanA gene, associated with vancomycin resistance, was found in Four vancomycin-resistant enterococci (VRE) isolates n =14 (28%), with a product size of 300 bp. The product size of E. faecalis endocardiatis antigen (efa) gene was 600 bp found in Eight isolates n=10 (80%). Quorum sensing genes (Las-R & Rhl-r) were identified in P. aeruginosa isolates associated with biofilm production. The product sizes were 700 and 600 bp, respectively. Twenty isolates were positive from Thirty-three isolates tested n=33(60%) for qouram sensing genes. The product size of the algD gene GDP mannose dehydrogenase in P. aeruginosa was 1 kb. Six MRSA strains were found positive for methicillin resistance (mec A) and pantovalentine leukocidine genes (Pvl) n=10 (60%) S. aureus isolates. The product sizes of mecA and Pvl were 400 and 450 bp, respectively. Conclusion: The uropathogenic strains isolated from diabetic patients carry a variety of virulence genes that contribute to the pathogenesis of UTI. Our study shows that UTI in diabetic patients is complicated by antimicrobial resistance, toxin production, adhesion mechanisms, and biofilm formation. The detection of virulence genes can help to develop better diagnostic markers for the detection of UTI pathogens. Keywords: Diabetes mellitus, Antibiotic resistance, Biofilms, Virulence, Quorum sensing. IntroductionDiabetes and UTIDiabetes mellitus (DM) significantly increases the risk of urinary tract infections (UTIs) due to several interconnected factors. People with diabetes, particularly women, are more prone to frequent UTI’s than those without diabetes. The heightened risk is primarily due to the changes in the Immune System changes associated with diabetes and anatomical factors, such as the shorter length of the urethra in women facilitating the entry of bacteria into the bladder (Boyko et al., 2005). In addition, diabetes can impair blood circulation, which in turn reduces the ability of white blood cells to effectively fight infections. Elevated blood glucose levels (hyperglycemia) may also affect the function of polymorphonuclear leukocytes, which are essential for response to infection. Studies have suggested that leukocyte dysfunction in diabetes may increase the risk of UTIs. Furthermore, many individuals with diabetes experience bladder dysfunction, leading to poor bladder contraction. These changes result in urine retention, creating an environment in which bacteria can thrive, further increasing the risk of UTIs. Diabetic women with UTI’s are also more susceptible to complications such as pyelonephritis and frequently require hospitalization when compared with non-diabetic women (Scholes et al., 2000). These factors like immune system impairment, anatomical susceptibility, and bladder dysfunction, combine to make diabetic patients vulnerable to UTIs. Common pathogens causing UTI include Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus mirablis, Enterococcus faecalis, Staphylococcus aureus, and Staphylococcus saprophyticus. Virulence genes play a crucial role in determining the severity and spread of UTIs in both diabetic patients and individuals without diabetes. Despite the known association between diabetes and increased UTI risk, the specific mechanisms by which diabetes predisposes individuals to recurrent and severe UTI’s remain inadequately understood. The role of virulence genes in uropathogenic bacteria, predominantly in the context of diabetic patients, is not fully elucidated. Although leukocyte dysfunction and bladder dysmotility have been suggested as contributing factors, the exact clinical implications of these alterations in patients with diabetes and UTIs are unclear (Brauner et al., 1993). There is a need for more comprehensive studies that explore the interplay between diabetes, bacterial virulence factors, and host immune responses to better understand and manage UTIs in this vulnerable population. The aim of this study is to provide a novel insight into the detection of various determinants of Virulence in uropathogens not elucidated by previous studies. Significance of Virulence genes in uropathogensEscherichia coliThe most common cause of UTIs in both men and women with, or without DM is E. coli. Some studies have noted that lower UTI’s caused by this organism are more frequent in patients with diabetes than age-matched controls without diabetes. Uropathogenic E. coli (UPEC) is the primary cause of UTI due to its colonization of the gut and genitourinary tract (Behzadi, 2018). Over 80% of community-acquired UTI’s are attributed to uropathogenic E. coli (UPEC) (Hooton and Stamm, 1997). Physiologically, E. coli is a highly adaptable organism that can thrive in environments where glucose is the sole organic nutrient. Wild-type E. coli does not require additional growth factors and can metabolically convert glucose into various macromolecules essential for cell structure. One of the key virulence factors produced by UPEC is the cytotoxic necrotizing factor type 1 (Cnf1), a 115-kDa chromosomal-encoded toxin. Cnf1 plays a crucial role in activating Rho GTPases and catalyzes the deamidation of small GTPases like RhoA (Flataue and Boquet, 1997). Epidemiological studies have suggested a link between Cnf1 production by E. coli and UTI, as well as the cytopathic effects observed in cultured urinary tract cells, indicating that Cnf1 may be a significant UPEC virulence factor (Johnson, 1991). However, the potential relationship between the virulence genotype of E. coli strains in diabetic patients with UTI has not yet been fully explored. This study aimed to compare E. coli isolates from patients with diabetes with symptomatic and asymptomatic bacteriuria, focusing on the phenotypic and genetic determinants that might serve as predictors of bacteremia to form better treatment strategies. Pseudomonas aeruginosaPseudomonas aeruginosa is a significant uropathogen and a major cause of complicated UTIs, especially in Immuncompromised individuals such as those with diabetes (Shigemura et al., 2003). Because of its clinical importance in a variety of infections, P. aeruginosa is considered an important pathogen in UTIs. Diabetic patients who are Immuncompromised are at a higher risk of developing infections caused by P. aeruginosa. This bacterium's prominence as a potent pathogen can be attributed to several physiological factors: such as minimal nutritional requirements, ability to target Immuncompromised hosts, capacity to develop resistance through multiple known mechanisms, and its potential to infect nearly any body site, with extreme tolerance to a wide range of physical conditions. P. aeruginosa is known for its opportunistic behavior, which has led to extensive research into its pathogenic mechanisms. One of the key factors in virulence is the secretion of alginate, an exopolysaccharide, during various infections. Although the genetics, biosynthesis, and regulation of alginate are well understood, unanswered questions remain regarding its role in biofilm development and its potential as a therapeutic target. The alginate biosynthetic pathway has been the focus of research for many years, and several comprehensive reviews have been published on the topic (Ramsey and Wozniak, 2005). The algA, algC, and algD genes of P. aeruginosa encode enzymes necessary for the synthesis of the alginate precursor guanosine diphosphate (GDP) mannuronic acid. Once mannuronic acid is synthesized, it is polymerized and transported across the inner membrane by a hypothesized combination of the alg8 and alg44 genes. Alginate production, a key component in the formation of P. aeruginosa biofilms, was first observed in mucoid strains isolated from patients with cystic fibrosis. More recent research confirmed that P. aeruginosa also forms biofilms in vivo (Singh et al., 2000; Miranda et al., 2022; Hemmati et al., 2024), which are essential for its virulence in various infections, including UTIs. In this study, we investigated the extensive role of the algD gene in the virulence of P. aeruginosa in UTIs. P. aeruginosa employs sophisticated cell-to-cell signaling systems to regulate the expression of its virulence factors. The first such system identified was the las system, which controls the expression of LasB elastase and other extracellular virulence factors, including LasA protease and exotoxin A. The las system consists of the lasI gene, which encodes the autoinducer synthase responsible for the synthesis of 3-oxo-C12-HSL (N-[3-oxododecanoyl]-L-homoserine lactone), and the lasR gene, which encodes a transcriptional activator protein. Activation of the lasI promoter by the LasR/3-oxo-C12-HSL complex leads to a rapid increase in autoinducer synthesis, enhancing the production of virulence factors. In addition to the las system, P. aeruginosa possesses a second cell-to-cell signaling system known as the rhl system, which regulates the production of rhamnolipids. This system comprises rhlI, which is responsible for synthesizing the autoinducer C4-HSL (N-butyrylhomoserine lactone), and rhl-R, which encodes a transcriptional activator protein. The rhl system controls the expression of the rhlAB operon, which encodes the rhamnosyltransferase required for rhamnolipid production. Moreover, the rhl system is essential for the optimal production of LasB elastase, LasA protease, pyocyanin, cyanide, and alkaline protease. Similar to the las system, the rhl system, which is sometimes referred to as the VSM (virulence secondary metabolites) system, plays a crucial role in regulating the expression of various extracellular virulence factors in P. aeruginosa. Through this study, we sought to deepen our understanding of how these signaling systems and the algD gene contribute to the virulence of P. aeruginosa in UTIs, with the goal of identifying potential therapeutic targets for more effective treatment. Enterococcus faecalisIn this study, we also investigated similar virulence factors in E. faecalis. Although much remains unknown about the specific factors E. faecalis utilizes to cause infections in the urinary tract or the host's response to this bacterium, research has increasingly focused on identifying the factors on both the host and pathogen sides that contribute to UTIs caused by E. faecalis (Guzmàn et al., 1989). Enterococci are among the leading causes of bacteremia in nosocomial infections, including UTIs evidence suggests that biological markers, such as the efa gene, may play a critical role in modulating the expression of enterococcal virulence-associated genes, particularly in the urinary tract environment (Shepard and Gilmore, 2002). Recent studies have also indicated that E. faecalis is frequently acquired through cross-infection from other patients, contributing to its persistence in healthcare settings. A significant challenge in treating E. faecalis infections is its escalating resistance to antimicrobials, leading to the emergence of vancomycin-resistant enterococci (VRE). This resistance presents a major therapeutic challenge, particularly with the VanA phenotype, which is associated with glycopeptide resistance (Dutka-Malen et al., 1995). In this study, we focused on the VanA phenotype to better understand its role in the resistance profile of E. faecalis and its implications for treatment strategies for UTIs. Staphylococcus aureusIn recent years, there has been an increasing incidence of UTI caused by S. aureus. About 0.5%–6% of UTI’s are caused by S. aureus; however, the bacterium is mistaken as a contaminant in positive urine cultures, which leads to the under treatment of the infection resulting to a life-threatening illness. The frequency of MRSA has exhibited an increase in causing UTI’s over 70 years of age in Japan, the United States, and France (Baba-Moussa et al., 2008). S. aureus has been associated with UTI in women with diabetes as well as in patients with nosocomial Infections. Methicillin-resistant S. aureus (MRSA) is resistant to a large group of antibiotics called beta-lactams, which include penicillin and cephalosporins. S. aureus has developed an ability to survive the effect of beta-lactamase, thus resistant to beta-lactam antibiotics, including methicillin, dicloxacillin, nafcillin, and oxacillin. MRSA is especially troublesome in hospital-associated nosocomial infections. Few studies have defined the epidemiological or microbiological factors responsible for the emergence or the spread of MRSA. We examined the molecular etiology of S. aureus in causing UTI among DM patients. In this communication, we report the presence of two virulent genes, mec A, and the Pantovalentine leukocidin gene (PVL) which may play a role in UTI. Although diversity and variation in their genomic and antibiogram backgrounds exist, virtually all of these newly emerging community-associated MRSA (CA-MRSA) strains carry the PVL virulence genes and possess a novel small mobile staphylococcal cassette chromosome mec (SCCmec) type IV or V genetic element that harbors the methicillin resistance (mecA) gene and is more easily transferred to other strains of S. aureus than the larger SCCmec types (types I to III) that are prevalent in HA-MRSA strains. PVL is a bicomponent leukocidin encoded by two co. transcribed genes, namely, lukS-PV and lukF-PV (lukS/F-PV), which reside on a prophage and cause leukocyte destruction and tissue necrosis. Until recently, genes encoding PVL were infrequently encountered, being noted in <5% of S. aureus isolates worldwide. However, they are found in a very high proportion of newly emerging CA-MRSA strains, with rates of 77%–100%, as reported in various studies. We investigated S. aureus isolates in our study for the identification of antibiotic-resistance genes such as those responsible for methicillin resistance (mecA). This demonstrates that these genes are involved in community-acquired UTI. Materials and MethodsStudy designA total of 1000 urine samples were collected from both diabetic and non-diabetic patients from higher and lower socioeconomic status who were attending the Diabetology Department at Bangalore Hospital, India. The demographic distribution of the patients selected for the study is presented in Table 1. Urine samples of patients with type 1 diabetes (100) were obtained from a Diabetic Center located in Bangalore city. Self-prepared questionnaires were given to patients with diabetes to obtain information on age, history of urinary frequency, abdominal pain, Hypertension, Cardiovascular diseases, Lipidemia, or any previous history of Infections. Midstream or clean-catch urine samples were collected in sterile containers and processed in the laboratory within two hours of collection. 1 µl of each urine sample was inoculated onto sterile Petri dishes using a calibrated inoculation loop and streaked onto HiChrome UTI agar (Himedia Labs India Pvt Ltd). The plates were then incubated overnight at 37°C. Colonies were identified based on color produced on Hi-Chrome UTI Agar. A colony count of 100,000 cfu/ml was considered significant for UTI. Non Lactose fermenters (NLf’s) were identified using standard biochemical tests. Isolated colonies of E. coli, P. aeruginosa, Enterococcus faecali, and S. aureus were subsequently transferred into Eppendorf tubes containing semisolid media, which served as stock cultures for further analysis. Antibiotic sensitivity testingThe antibiotic sensitivity of UTI isolates was assessed using the Kirby–Beur or disc diffusion method in accordance with the CLSI guidelines. A loopful of 3–4 colonies of test organisms (S. aureus, E.coli, P. aeruginosa, and E. faecalis ) were selected from the pure culture plates and transferred into 5 ml of sterilized Brain heart infusion broth (Himedia Labs Pvt., Ltd.). The tubes were incubated at 34°C for 24 hours to reach 0.5 Mcfarland standard for turbidity. A nontoxic swab was dipped into the Standardized inoculum and rotated. The fully soaked swab was pressed firmly against the upper wall of the tube to express excess fluid. The entire surface of the plate was swabbed three to four times by turning the plate at 60cº angle between each streak. The inoculum was allowed to dry for 5–15 minutes with the lid in place. Following the aseptic technique, antibiotic discs (Himedia Pvt., Ltd.) were placed on the surface of the agar using a sterile forcep. The discs were placed at a distance of 24 mm apart. Plates were incubated at 37 cº for overnight incubation and results were tabulated according to the complete zone of inhibition observed to the nearest millimeter. The gram positive and gram negative isolates were tested for cephalosporins, aminoglycosides, fluroquinalones, and glycopeptides as recommended CLSI standards. Positive isolates of VRE, E. coli, and multidrug-resistant P. aeruginosa were further analyzed for the detection of Virulence genes by PCR. The primers were commercially synthesized using the sequences of specific genes as described in previous studies and were compared with the gene sequences in Gene Bank Table 2. Fifteen isolates of E. coli were selected for the detection of Cnf gene followed by ten isolates E. faecalis for the detection of the efa gene and 14 isolates for the detection of the Van A gene. Thirty-three isolates were selected for the detection of the biofilm genes Las-r and Rhl-r in P. aeruginosa. Ten isolates of S. aureus were selected for the detection of mec A and Pvl. genes. Table 1. Sociodemographic distribution of patients studied.
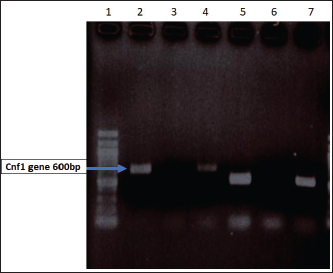
Plasmid DNA extraction and purificationPlasmid DNA from UTI isolates was purified using the method described by P. courvalin (Antibiotic Resistance Techniques, 5th edition, Pasteur Institute, 2008) A loopful of stock culture of each UTI isolate was inoculated into 5 ml of Brain Heart Infusion (BHI) broth and incubated overnight at 37°C under appropriate conditions. 2 ml of the overnight cell suspension was centrifuged at 15,000 rpm for 5 minutes in a refrigerated centrifuge. The resulting pellet was re-suspended in 100 µl of TE buffer (Tris-HCl 10 mM, pH 8.0; EDTA 1 mM). For gram-positive Enterococcus species, the pellet was further resuspended in 100 µl of TGE-lysozyme and incubated at 37°C. A 200 µl of NaOH-SDS solution was added to the mixture, which was gently vortexed by inverting the tube 4–5 times. The mixture was allowed to stand on ice for 5 minutes. 150 µl of potassium acetate solution was added, and the tube was placed on ice for an additional 10 minutes. Phenol-chloroform extractionA 200 µl of Tris-saturated phenol (Bangalore Genei India Pvt., Ltd.) and 200 µl of chloroform were added and well mixed by vortexing. The mixture was centrifuged for 3 minutes, and 400 µl of the upper aqueous layer (supernatant) was carefully transferred to a fresh tube without disturbing any white residues. The supernatant was washed with 800 µl of absolute ethanol and then centrifuged. The pellet was washed with 70% ethanol and centrifuged for an additional 5 minutes. The pellet was air-dried and resuspended in 100 µl of TE buffer (Tris-HCl 10 mM, EDTA, pH 8.0) with 1 µl of RNAse (Chromous Biotech Pvt Ltd). Detection of the cytotoxic necrotizing factor type 1 (Cnf) gene in E .coli by PCRCytotoxic necrotanizing factor type 1 (cnf) is a virulent gene found in many strains of uropathogenic E. coli. Primers for this gene were synthesized according to the sequence and PCR method described (Rippere-Lampe et al., 2001). The primer sequences for the cytotoxic gene (Table 3) were added in the reaction method prepared according to the method mentioned above for all consecutive PCRs. The PCR Reaction was set at the following cycling conditions in a thermocycler for denaturation: 30 consecutive cycles of 1 minutes at 95°C, 1 minutes at 50°C, and 3 minutes at 72°C with a final extension at 72°C for 10 min. All PCR products were examined on 1.5% agarose gels (Fig. 1). Detection of Van A gene Enterococcus faecalis using polymerase chain reactionPrimers for two virulent-associated genes VanA associated with Vancomycin resistance in vancomycin-resistant Enterococcus (VRE) and a cell wall adhesion factor (efa) which is expressed in urine were synthesized according to the sequences and PCR methods described by (Shepard and Gilmore, 2002; Baba-Moussa et al., 2008; Bell et al., 1998; Salah et al., 2008) . PCR conditions for efa gene were accomplished by a PCR thermocycler (MJ research- INC, USA) and were obtained at 15 minutes initial enzyme activation, DNA denaturation step at 95°C followed by 35 consecutive cycles at 94°C for 20 s, 68°C for 45 s, and 72°C for 15 s according to the primer sequences discussed in Table 2. The cycling conditions followed were initial denaturation at °C for 10 min, followed by annealing at 94°C for 1 min, 50°C for 1 min, and extension at 72°C for 1 minutes for 40 cycles and 72°C for 5 mins for the vanA gene. All PCR products were examined on 1.5% agarose gels (Figs. 2 and 3). Table 2. Primer sequences for PCR assays of target genes of UTI isolates.
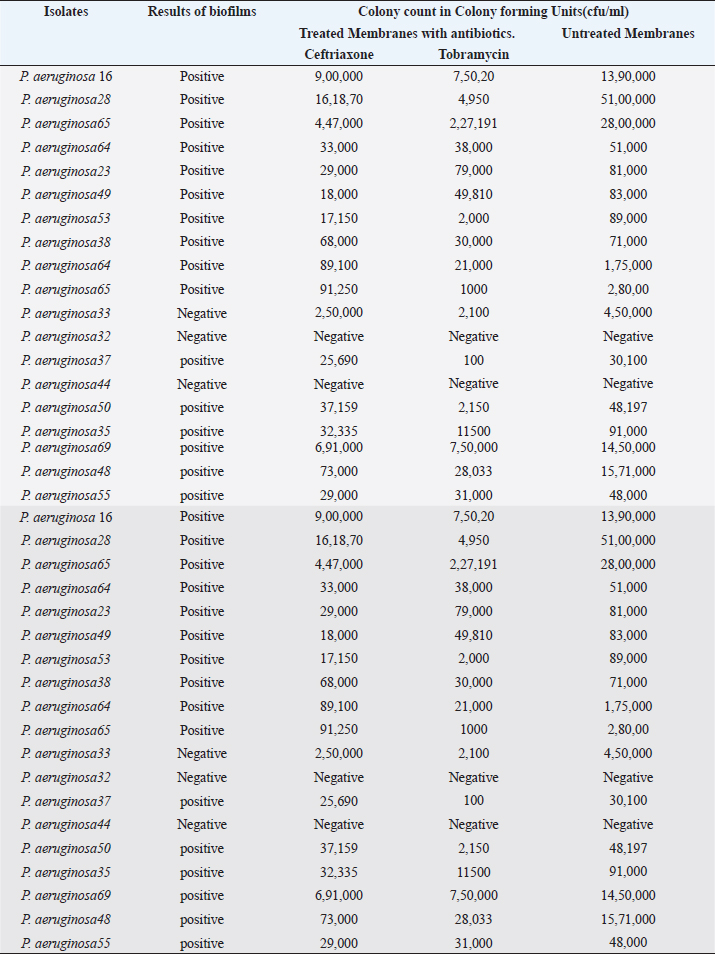
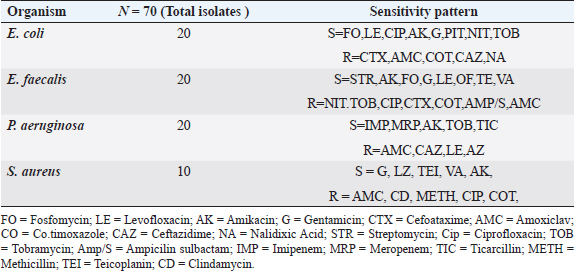
Detection of AlgD gene in Pseudomonas aeruginosa using polymerase chain reactionThe AlgD gene was amplified using primers according to the sequence described (Lanotte et al., 2004). Table 2 using 1.0 µl of template DNA as extracted through mini prep, AlgD Forward primer, 2.0 µl algD Reverse primer 2.0 µl, dNTPs 2.5mM (Chromous biotech pvt LtD) 2.0 µl Taq Assay buffer 10X (chromous Biotech Ltd India) 5.0 µl, Taq polymerase (Chromous biotech pvt Ltd India) 0.5 µl, nuclease-free Water 37.5 µl following the PCR conditions as follows: initial denaturation at 94cº for 5 minutes, annealing at 50cº for 45seconds, extension at 72cº for 1minute and single final extension at 72cº for 5 mins and analyzed on 2% agarose gel Fig 4. Detection of quorum sensing genes (las-r and rhlR) in P. aeruginosa biofilmsOligonucleotide primers for las-r and rhlR (Chromous Biotech Pvt ltd) expression genes were used to study the quorum sensing genes. Amplification cycles were performed for an initial denaturation time of 1 minutes at 94°C, annealing of primers for 1 minutes at 52°C, and primer extension for 1.5 minutes at 72°C for 30 cycles. Agarose gel (2.0%) (Fig. 5) was used to examine the products after PCR using the primer sequences discussed in Table 2. Identification of Pseudomonas aeruginosa using 16rDNA sequencingGenomic DNA was isolated from the pure culture of the test organisms. The rDNA fragment was amplified using a high-fidelity polymerase chain reaction. The PCR product was sequenced (Chromous Biotech Pvt Ltd India) bidirectionally using forward and reverse primers, and the sequence data was aligned and analyzed for homology with the sequences available in GenBank with NCBI Accession No P. aeruginosa AL98; AJ249451, 42A2 NCBI 40045; AJ309500 0.996 Pseudomonas sp.; OLB-1, AJ387904. Growing and analyzing static biofilms in P. aeruginosa isolatesA colony biofilm assay was performed on P. aeruginosa isolates using a previously described method (Merritt et al., 2005). Five isolates of P. aeruginosa were selected for the study. A loopful of the culture was inoculated into 5 ml of Luria–Bartini broth and grown overnight at 37cº. Using sterile forceps, a 25 mm nitrocellulose membrane with a pore size of 0.22 um was positioned in a sterile Petri plate approximately 30 cm apart from the UV light source in a sterile environment. Diluted stationary-phase cultures (step 1) in the appropriate medium to an OD600 of 0.05 (~1.64 × 108 cfu/ml) were used. The nitrocellulose membranes were placed with their shiny side up on an agar medium plate not containing antibiotics, and inoculated with 5 μl diluted culture. The procedure was repeated for all remaining membranes from (step 2), inoculating up to six membranes on the same agar plate. When the liquid on the membranes had dried, the plates were incubated upright at the appropriate temperature for 24 hrs. Using sterile forceps, each membrane was gently lifted off of its agar plate and transferred to a fresh agar plate at up to six membranes per plate. The plates were incubated for 24 hr at the appropriate temperature (Fig. 6). Biofilm growth was analyzed by aseptically transferring the membranes in 15ml of tubes containing 10ml of sterile phosphate buffer saline. The tubes were capped, and the samples were vortexed to detach all the bacteria. A dilution series of the vortexed samples was prepared, and each dilution series was prepared on separate agar plates. The plates were incubated overnight, and the colony count was determined using the average number of colony-forming units (cfu) per membrane. A similar method was followed with membranes supplemented with and without antibiotics (Ceftriaxone and Tobramycin) Table 3. PCR analysis of virulent genes in S. aureusThe virulent genes mecA and Pvl were investigated using PCR in S. aureus. Six MRSA isolates of S. aureus ere investigated in this study. The primer sequence was followed by the method described in Table 2. PCR was performed in a 44µL reaction mixture containing 1.5µl of dNTPs (2.5 mM), Taq buffer 5 µl, 1 µl Mgcl2, 1 µl of Taq polymerase, 1 µl (chromous Biotech Pvt LtD, India ) of each primer, and 1 µL of bacterial DNA template. The PCR conditions began with initial denaturation at 95cº for 45 sec, extension at 60cº for 1 min, and final extension at 72cº for 2 min. The amplification conditions for the Pvl gene were denaturation at 94c for 30 sec, annealing at 1 minutes at 55c, elongation at 72c for 1 min, and final elongation at 72cº for 1 minutes. The product size was determined using 2% agarose gels (Fig. 7). Agarose gel electrophoresis of the Plasmid DNA and PCR productsFive microliters of the purified plasmid DNA were loaded directly onto an agarose gel, along with a Lambda DNA/Hind III digest molecular marker (Chromous Biotech Pvt Ltd). Lambda DNA, digested with Hind III, generates eight fragments ranging from 1215 bp to 23,130 bp. The concentration of the marker is 200 ng/µl in a 1% agarose gel. This method ensured the isolation of high-quality plasmid DNA suitable for further analysis and experimentation. All PCR products were analyzed in 1.5%–2% of Agarose gels depending on the size of the PCR product. Data analysisData was tabulated in percentages (frequencies) using the statistical software SPSS Version 26.0 (SPSS Inc., Chicago, IL). continuous variables were summarized in the form of means, and standard deviations and categorical variables were summarized as percentages. Chi-square test or Fisher's exact test, whichever appropriate, was applied for comparing categorical variables. A p-value of less than 0.05 was considered statistically significant at 95% confidence interval. Ethical approvalSince the urine samples were collected directly from the microbiology departments of various hospitals after the required approvals, no ethical approval was mandatory, as decided by the advisory committee. ResultsAmong the patients with diabetes, 140 were males and 110 were females. The prevalence of uropathogens in both sexes was E.coli 46 (32.9%) 28 (25.5%), E. faecalis 44 (31.4%), 32(29.1%) P. aeruginosa 2 (1.4%) 10 (9.1%), and S. aureus 26 (18.6%) 22 (20%), respectively. 22 males (15.7%) and 18 females (16.4%) were negative for UTI. In the higher Socioeconomic group with diabetes, the prevalence of E.coli 18(13.8%) 13(10.8%), E. faecalis 32(24.6%), 36 (30.0%), and P. aeruginosa 30 (23.1%) was observed in both males and females. The incidence of Uropathogens in patients with Type 1 diabetes was E.coli 22(44%) in males and 15(30%) in females, E. faecalis 10(20%), 20(40%), and S. aureus 14(28%), 8(16%), respectively. 8% of males and 14% of females had no evidence of UTI. The prevalence of uropathogens in non-diabetic patients was significantly lower than that in diabetic patients E.coli (8.4%) and E. faecalis (20%). There was no prevalence of P. aeruginosa in the non-diabetic group. The prevalence of S. aureus was 10.8%. 60.88% of non-diabetic patients were culture negative for UTI. The above results of the prevalence of UTI among diabetic patients are published elsewhere (Saleem et al., 2011). Twenty isolates of each E. coli strain, P. aeruginosa, E. faecalis, and ten isolates S. aureus (Total Seventy) were tested for antibiotic sensitivity, and the results were recorded as Table 4. UPEC produces a number of virulence-associated factors, which include P fimbriae hemolysins, aerobactin, and Cytotoxic necrotinizing factor type 1Cnf1. The epidemiological studies linking Cnf1 production by E. coli with urinary tract disease and the cytopathic effects of Cnf1 or cultured urinary tract cells are suggestive of a role for the toxin as UPEC virulence factor. We investigated this gene in 15 E. coli. isolates. Amplification products of the expected size 600 bp for CNF1 were detected in Seven Isolates (46%). Therefore, the specificity and sensitivity of the two pairs of oligonucleotide primers designed in this study were 100%. The PCR protocol described here permits rapid and accurate detection of the CNF1 gene in E. coli strains isolated from urine samples of patients with diabetes. To confirm the specificities of the various PCR primers listed for VanA in representative VRE isolates were analyzed as described in Materials and Methods. Clear PCR products of an expected size of 300 bp for vanA were obtained. There was 100% agreement between the PCR results and previously published genotypes, and phenotypes. Four isolates of VRE were positive for the presence of VanA among the 14 tested isolates (28%). Similar results were obtained for the efa gene in E. faecalis. Out of the 10 tested isolates, 8 (80%) were positive in diabetic urine samples. The product size in the case of efa gene was 600 bp and VanA gene was 300 bp. Approximately 1.5-kb 16sRDNa fragment of P. aeruginosa was amplified using high-fidelity PCR. The sequence data were aligned and analyzed to identify the MDR strain of P. aeruginosa using the sequence available in GenBank. The strain for identification was most similar to P. aeruginosa GenBank entry AJ249451). The prevalence of virulence genes encoding alginate (algD) was determined by PCR. The gene was amplified with primers described in the materials and methods.1 kb PCR product was obtained following successful amplification and compared with the 5-kb ladder (Chromous Biotech pvt India ltd). However, only one isolate of MDR P. aeruginosa was investigated. The sequence of the AlgD gene was also submitted to the gene bank with accession number MT916847. The quorum sensing genes of P. aeruginosa , las-R, and rhl –R underwent PCR amplification as described in the materials and methods. Twenty isolates (60%) were found positive for thirty-three isolates of P. aeruginosa. The product sizes of were found to be 700 bp and 600 bp, respectively, in each isolate, which was less than the expected size, of the genes as mentioned previously in earlier studies. P. aeruginosa isolates exhibited complete biofilm formation in all isolates. The colony count of P. aeruginosa isolates treated with ceftriaxone indicated a higher colony count, thus reflecting resistance to cephalosporins. In addition, resistant mutants may be indicated in biofilm producers of P. aeruginosa isolates when treated with Tobramycin. However, there was a more significant colony count observed in untreated membranes. Of the 20 isolates, 16 were biofilm producers out of 20 Isolates (Table 3). Six MRSA strains (60%) were found to be positive for methicillin resistance (mec A) and pantovalentine leukocidine genes (Pvl) when tested out of 10 isolates Table 5, Figure 8. Table 3. Biofilm producers and colony count of Pseudomonas aeruginosa.
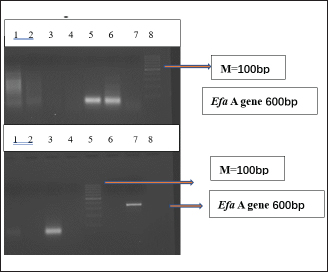
Fig. 1. Distribution of Cnf gene E. coli Isolates. Lane 1 Molecular Marker 100bp ladder. Lane 2 Positive amplification band represents the presence of Cnf; Lanes 4, 5, and 7 the gene size was approx. 580 bp.
Fig. 2. Distribution of efaA among E. faecalis isolates. M, 100 bp DNA marker; Lane 1 and Lane 2: the band represents the presence of efaA gene (600 bp).
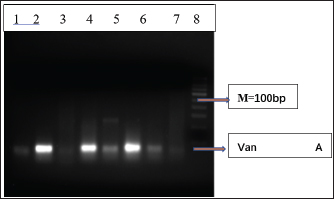
Fig. 3. Distribution of the Van A gene among the E. faecalis isolates. M, 100-bp DNA marker; Lane 1 (1–7) represents the presence of Van A gene (300 bp).
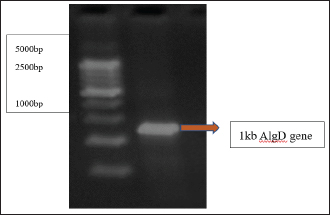
Fig 4. AlgD gene in MDR P. aeruginosa isolate PCR band of 1,000 bp represents the presence of AlgD gene on 2% agarose gel after comparison with 5,000 bp molecular marker.
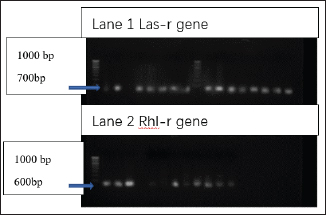
Fig. 5. PCR amplification bands representing the presence of las-r and rhlR biofilm genes in P.aeruginosa isolates (700 and 600 bp). Lane 1: 100b bp DNA marker, Positive amplification of the las-r gene in 3–9 (14 samples). Lane 2 : 100 bp DNA marker,;positive amplifications of rhl-R gene were observed sample 1–3, sample 7, 9, 10, and 11 (8 samples of P. aeruginosa). DiscussionMicrobial pathogenesis involves studies that are traditionally based on evidence of virulence properties, such as the ability of a pathogen to elaborate toxins, adhere to, and invade host cells, causing tissue damage, and disrupting the normal immune system. These changes are prominent and advanced in the etiology of UTI. Since UTI is the most frequently diagnosed urologic disease in patients with DM, it is equally important to study the factors directly related to the virulence of UTI pathogens. UPEC is the major causative agent of UTI (Holden et al., 2006). UTIs are considered acute, self-limiting infections despite the prevalence of recurrent infection two or more times within months of a primary infection. The currently recognized virulence factors such as Cnf gene accounts for only a fraction of the total virulence of wild-type strains, suggesting that other as-yet-unidentified properties that are important determinants of virulence await discovery and characterization. Because limited studies have identified the role of Cnf gene in UPEC in vivo, this study investigated this gene from the bacterial isolates grown in vitro. This simple method of PCR detection and observation suggests an intriguing new line of study for investigators interested in the pathogenesis of UTI and also reflects the fact that cnf gene can be directly involved in the etiology of ascending UTI in patients with diabetes. However, very few studies have described the epidemiology and clinical importance of VRE in urinary isolates. Our study demonstrated that VRE urinary isolates are present in patients with diabetes and a history of UTI. Our data indicates that despite the predominance and widespread distribution of the vanA resistance determinant, the vanB genotype has become well established and shows remarkable stability in the diabetic population (Dahl et al., 1999). Glycopeptide susceptibilities and PCR revealed that the VanA genotype is widely disseminated among VREs isolated from patients with DM. Because recurrent UTI’s are more frequent in patients with DM, it is important to treat UTIs with VRE in the future. Virulent factors of E. faecalis include adherence to host tissue, invasion, and abscess formation, modulation of host inflammatory responses, and secretion of various products that enhance infection. Therefore, more investigation on potential virulence factors like efa gene of E. faecalis, would be useful in understanding their role in UTI. Moreover, clinical isolates of E. faecalis recovered from UTI isolates can exhibit resistance to conventional treatment regimens recommended for UTI. Thus far, efa has been only associated with the virulence of endocardiatis; however, less evidence is available on the role of this gene in causing acute UTI. This study showed that all E. faecalis isolates were associated with UTI, especially in patients with diabetes, as they carried the efa gene. Our study thus indicates that efa gene also has a potential role in the pathophysiology of UTI, as this gene can be responsible for bacterial adhesion, despite the fact that only a few UTI isolates were found positive for carrying this gene. Our results revealed a significant difference between the proportion of MDR P. aeruginosa strains isolated from diabetic patients belonging to the Lower socioeconomic status expressed one of the key virulence genes. Numerous reports have documented that the rise in MDR nosocomial P. aeruginosa continues to threaten hospitalized patients despite various measure, including isolation techniques and antibiotic de-escalation therapy. Whether MDR P. aeruginosa strains necessarily express a more virulent phenotype remains controversial. This study initiated the isolation of MDR P. aeruginosa clinical strains for their ability to disrupt the integrity of human cultured intestinal epithelial cells and correlated these findings to related virulence phenotypes such as adhesiveness, motility, biofilm formation, and cytotoxicity, which have been previously documented in earlier studies (López-Jácom et al., 2019). These strains were characterized and found to harbor the algD gene and to display high swimming motility and adhesiveness. We observed a significantly higher spread of the virulence genes las-R and rhl-R among MDR P. aeruginosa isolates. Our experiments, which included analysis of biofilms among the P. aeruginosa isolates, significantly reflected high antimicrobial resistance to β-lactams and aminoglycosides.
Fig. 6. Colony biofilm assay of Pseudomonas aeruginosa on nitrocellulose membrane.
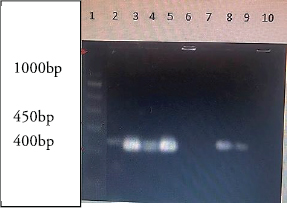
Fig. 7. Lane 1 is the 1,000 bp molecular size standard. Band of 400 bp represents mec A amplification in MRSA isolates.
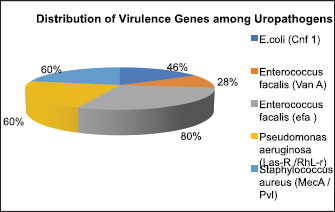
Fig. 8. Distribution of virulence genes among uropathogens. Table 4. Antibiotic sensitivity pattern of UTI Isolates.
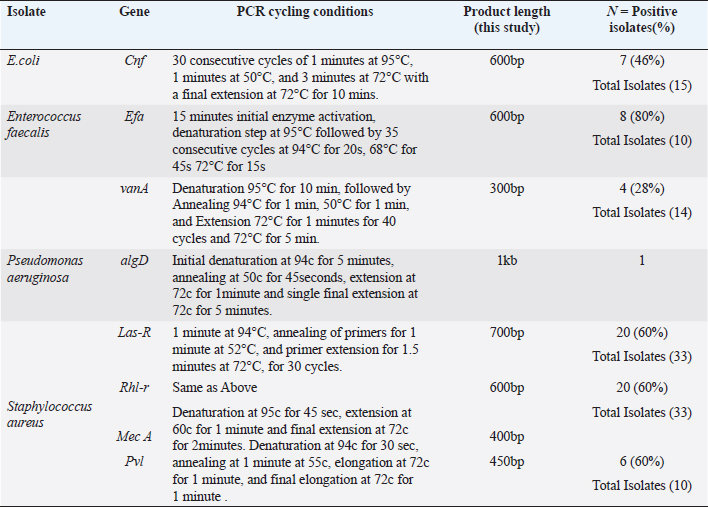
Table 5. PCR cycling conditions and percentage distribution of virulence genes among different species of Uropathogens.
The present study revealed a higher frequency of spread of quorum sensing and biofilm-associated genes among P. aeruginosa. Simultaneous determination of virulence factors and antimicrobial resistance is a contemporary approach for examining the microbiological aspects of infections caused by P. aeruginosa. The presence of more than one virulence factor in our UTI isolates were notable which reflects on the wide distribution of these virulence factors that cause frequent UTIs. The similarities of virulence factors in our UTI isolates with previous studies suggest that UTI pathogens are more aggressive. A larger study using alternate typing methods and UTI isolates from widely different areas and types of UTIs may be beneficial for the overall interpretation of the data. However, bias due to origin from a single state was not readily evident, as the isolates were quite diverse in terms of serogroups, genotypes, and phylogenetic groups. In fact, UTI pathogens that originated from patients with diabetes with lower socioeconomic status were well represented in several virulence genes. Their propensity for causing such diseases relates to their possession of many virulence-associated traits, including certain serogroups, adhesins, iron acquisition systems, toxins, protectins, and invasins, that enable them to grow and cause disease in these host environments, as published by us elsewhere. Consequently, it seems reasonable that these UTI isolates may show similar lifestyle adaptations, which, in turn, may enable uropathogens to cause more frequent UTIs in patients with diabetes. Further research is necessary to determine UTI-causing pathogens that serve as reservoirs of virulence genes that contribute to uropathogenesis. ConclusionVirulence genes play a pivotal role in the pathogenesis of UTIs, contributing to antimicrobial resistance, adhesion, and biofilm formation. While previous studies have primarily focused on Uropathogenic E. coli (UPEC), this study expands the scope by investigating virulence factors in other notable uropathogens responsible for UTIs, particularly in patients with DM who are at increased risk for recurrent infections. Our study shows that Molecular studies are valuable in understanding the genetic variations in a pathogen; however, they cannot assist in adjusting existing treatment strategies. Therefore, molecular studies can provide a platform for understanding the circulating strains and for developing better surveillance programs and Infection prevention strategies. Our study demonstrated the utility of virulence genes as diagnostic markers in MDR uropathogens. Conflict of interestThe authors have no conflict of interest to declare and all authors have contributed equally. Authors’ contributionsBD conceptualized the study, MS performed the experimental work and wrote the manuscript, and MA analyzed the sequenced data and submitted the sequence for accession Number in GenBank. ReferencesBaba-Moussa, L., Anani, L., Scheftel, J.M., Couturier, M., Riegel, P., Haikou, N., Hounsou, F., Monteil, H., Sanni, A. and Prévost, G. 2008. Virulence factors produced by strains of Staphylococcus aureus isolated from urinary tract infections. J. Hosp. Infect. 68, 32–38. Behzadi, P. 2018. Uropathogenic Escherichia coli and fimbrial adhesins virulome. Urinary tract infection: result of pathogen strength or weakness virulence and host factors in diabetic patients. Diabet. Med. 10, 550–554. Bell, J.M., Paton, J.C. and Turnidge, J. 1998. Emergence of vancomycin-resistant enterococci in Australia: phenotypic and genotypic characteristics of isolates. J. Clin. Microbiol. 36, 2187–2190. Flataue, P. and Boquet, P. 1997. Cytotoxic necrotizing factor 1 from Escherichia coli : a toxin with a new intracellular activity for eukaryotic cells. Folia Microbiol. 43, 285–289. Boyko, E.J., Fihn, S.D., Scholes, D., Abraham, L. and Monsey, B. 2005. Risk of urinary tract infection and asymptomatic bacteriuria among diabetic and nondiabetic postmenopausal women. Am. J. Epidemiol. 161, 557–564. Bratu, S., Gupta, J. and Quale, J. 2006. The expression of the las and rh quorum sensing systems in clinical isolates of P. aeruginosa is not correlated with efflux pump expression or antimicrobial resistance. J. Antimicrob. Chemother. 58(6), 1250–1253. Brauner, A., Flodin, U., Hylander, B. and Ostenson, C.G. 1993. Bacteriuria, bacterial of the host. Croatia: InTechOpen, pp: 65–83. Cotar, A.I., Dinu, S., Chifiriuc, M.C., Banu, O., Iordache, C., Larion, C., Bucur, M., Dracea, O. and Lazar, V. 2008. Screening of molecular markers of quorum sensing in Pseudomonas aeruginosa strains isolated from clinical infections. Roum. Biotech. Lett. 13(3), 3765–3770. Dahl, K.H., South Simonsen, G., Olsvik, O. and Sundsfjord, A. 1999. Heterogeneity of the van B gene cluster of genomically diverse clinical strains of the vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 43, 1105–1110. Dutka-Malen, S., Evers, S. and Courvalin, P. 1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 33, 24–27. Gomroki, F., Mohammed, H.B. and Malla, S. 2015. Amplification of methicillin resistant gene (mecA) gene from the MRSA strains. Int. J. Pharm. Clin. Res. 7(3), 198–203. Guzmàn, C.A., Pruzzo, C., LiPira, G. and Calegari, L. 1989. Role of adherence in pathogenesis of Enterococcus faecalis urinary tract infection and endocarditis. Infect. Immun. 57(6), 1834–1838. Hassan, M., Flayyih, M. and Yaseen, N. 2015. Isolation and purification of CNF1 (Cytotoxic necrosis factor 1) produced from bacteria Escherichia coli and study its role against apoptosis in vitro. Iraqi J. Cancer Med. Genetics 8(2), 157–163. Hemmati, J., Nazari, M., Abolhasani, F.S. and Asghari, B. 2024. In vitro investigation of relationship between quorum-sensing system genes, biofilm forming ability, and drug resistance in clinical isolates of Pseudomonas aeruginosa. BMC Microbiol. 24, 99. Holden, N.J., Totsika, M., Mahler, E., Roe, A.J., Catherwood, K. andLindner, K. 2006. Demonstration of regulatory cross-talk between P. fimbriae and type 1 fimbriae in uropathogenic Escherichia coli. Microbiology 152(4), 1143–1153. Hooton, T.M. and Stamm, W.E. 1997. Diagnosis and treatment of uncomplicated urinary tract infection. Infect. Dis. Clin. North Am. 11(3), 551–581. Jo-Ann, M., John, M.C., Vicky, L., Sameer, E., Thomas, L., Wendy, H. and Kunyan, Z. 2006. Novel multiplex PCR assay for detection of the staphylococcal virulence marker panton-valentine leukocidin genes and simultaneous discrimination of methicillin-susceptible from resistant staph. J. Clin. Microbiol. 44(3), 1141–1144. Johnson, J.R. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 4, 80–128. Lanotte, P., Watt, S., Merghitti, L., Dartiguelongue, N., Rastegar-Lari, A., Goudeau, A. and Quentin, R. 2004. Genetic features of P. aeruginosa isolates from cystic fibrosis patients compared with those of isolates from other origins J. Med. Microbiol. 53(1), 73–81. López-Jácom, L.E., Garza-Ramos, G., Hernández-Durán, M., Franco-Cendejas, R., Loarca, D., Romero-Martínez, D., Nguyen, P.T.D., Maeda, T., González-Pedrajo, B., Díaz-Guerrero, M., Sánchez-Reyes, J.L., Díaz-Ramírez, D. and García-Contreras, R. 2019. AiiM lactonase strongly reduces quorum sensing controlled virulence factors in clinical strains of Pseudomonas aeruginosa isolated from burned patients. Front. Microbiol. 10, 2657. Merritt, J.H., Kadouri, D.E. and O’Toole Analyzing, G.A. 2005. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. 1, 1–25. Miranda, S.W., Asfahl, K.L., Dandekar, A.A. and Greenberg, E.P. 2022. Pseudomonas aeruginosa quorum sensing. Adv. Exp. Med. Biol. 1386, 95–115. Ramsey, D.M. and Wozniak, D.J. 2005. Understanding the control of P. aeruginosa alginate synthesis and prospectus for management of chronic infections in cystic fibrosis. Mol. Microbiol. 56(2), 309–322. Rippere-Lampe, K.E., O'Brien, A.D., Conran, R. and Lockman, H.A. 2001. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf1) attenuates the virulence of uropathogenic Escherichia coli. Infect. Immun. 69, 3954–3964. Sabharwal, N., Dhall, S., Chhibber, S. and Harjai, K. 2014. Molecular detection of virulence genes as markers in Pseudomonas aeruginosa isolated from urinary tract infections. Int. J. Mol. Epidemiol. Genet. 5(3), 125–134. Salah, R., Dar-Odeh, N., Abu Hammad, O. and Shehabi, A.A. 2008. Prevalence of putative virulence factors and antimicrobial susceptibility of Enterococcus faecalis isolates from patients with dental diseases. BMC Oral Health 8, 17. Saleem, M. and Daniel, B. 2011. Prevalence of urinary tract infection among patients with diabetes in Bangalore City. Int. J. Emerg. Sci. 1(2), 133–142. Scholes, D., Hooton, T.M., Roberts, P.L., Stapleton, A.E., Gupta, K. and Stamssin, W.E. 2000. Risk factors for recurrent UTI in young women. J. Infect. 182, 1177. Shepard, B.D. and Gilmore, M.S. 2002. Antibiotic-resistant enterococci: the mechanisms and dynamics of drug introduction and resistance. Microbes Infect. 4(2), 215–224. Shigemura, K., Arakawa, S., Sakai, Y., Kinoshita, S., Tanaka, K. and Fujisawa, M. 2006. Complicated urinary tract infection caused by Pseudomonas aeruginosa in a single institution (1999-2003). Int. J. Urol. 13(5), 538–542. Singh, P.K., Parsek, M.R. and Welsh, M.J. 2000. Quorum sensing signals indicate that lungs with cystic fibrosis are infected with bacterial biofilm. Nature 407, 762–764. | ||
| How to Cite this Article |
| Pubmed Style Daniel B, Shah MS, Andrabi SM. Utility of PCR for the molecular characterization of virulence genes in bacteria associated with urinary tract infections in diabetic patients. J Microbiol Infect Dis. 2025; 15(1): 19-31. doi:10.5455/JMID.2025.v15.i1.3 Web Style Daniel B, Shah MS, Andrabi SM. Utility of PCR for the molecular characterization of virulence genes in bacteria associated with urinary tract infections in diabetic patients. https://www.jmidonline.org/?mno=220151 [Access: January 25, 2026]. doi:10.5455/JMID.2025.v15.i1.3 AMA (American Medical Association) Style Daniel B, Shah MS, Andrabi SM. Utility of PCR for the molecular characterization of virulence genes in bacteria associated with urinary tract infections in diabetic patients. J Microbiol Infect Dis. 2025; 15(1): 19-31. doi:10.5455/JMID.2025.v15.i1.3 Vancouver/ICMJE Style Daniel B, Shah MS, Andrabi SM. Utility of PCR for the molecular characterization of virulence genes in bacteria associated with urinary tract infections in diabetic patients. J Microbiol Infect Dis. (2025), [cited January 25, 2026]; 15(1): 19-31. doi:10.5455/JMID.2025.v15.i1.3 Harvard Style Daniel, B., Shah, . M. S. & Andrabi, . S. M. (2025) Utility of PCR for the molecular characterization of virulence genes in bacteria associated with urinary tract infections in diabetic patients. J Microbiol Infect Dis, 15 (1), 19-31. doi:10.5455/JMID.2025.v15.i1.3 Turabian Style Daniel, Betty, Mehvish Saleem Shah, and Syed Mudasir Andrabi. 2025. Utility of PCR for the molecular characterization of virulence genes in bacteria associated with urinary tract infections in diabetic patients. Journal of Microbiology and Infectious Diseases, 15 (1), 19-31. doi:10.5455/JMID.2025.v15.i1.3 Chicago Style Daniel, Betty, Mehvish Saleem Shah, and Syed Mudasir Andrabi. "Utility of PCR for the molecular characterization of virulence genes in bacteria associated with urinary tract infections in diabetic patients." Journal of Microbiology and Infectious Diseases 15 (2025), 19-31. doi:10.5455/JMID.2025.v15.i1.3 MLA (The Modern Language Association) Style Daniel, Betty, Mehvish Saleem Shah, and Syed Mudasir Andrabi. "Utility of PCR for the molecular characterization of virulence genes in bacteria associated with urinary tract infections in diabetic patients." Journal of Microbiology and Infectious Diseases 15.1 (2025), 19-31. Print. doi:10.5455/JMID.2025.v15.i1.3 APA (American Psychological Association) Style Daniel, B., Shah, . M. S. & Andrabi, . S. M. (2025) Utility of PCR for the molecular characterization of virulence genes in bacteria associated with urinary tract infections in diabetic patients. Journal of Microbiology and Infectious Diseases, 15 (1), 19-31. doi:10.5455/JMID.2025.v15.i1.3 |