| Case Report | ||
J. Microbiol. Infect. Dis., (2024), Vol. 14(1): 30–33 Case Report Epstein-Barr virus-associated polyserositis, thrombocytopenia, and elevated transaminases in a young femaleRahul Kumar, Pooja Khosla, Vinus Taneja*, Rishikesh Dessai and Manuj SondhiDepartment of Internal Medicine, Sir Ganga Ram Hospital, New Delhi, India *Corresponding Author: Vinus Taneja. Department of Internal Medicine, Sir Ganga Ram Hospital, New Delhi, India. Email: card.neuro [at] gmail.com Submitted: 09/01/2024 Accepted: 07/03/2024 Published: 31/03/2024 © 2024 Journal of Microbiology and Infectious Diseases
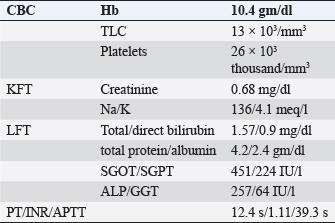
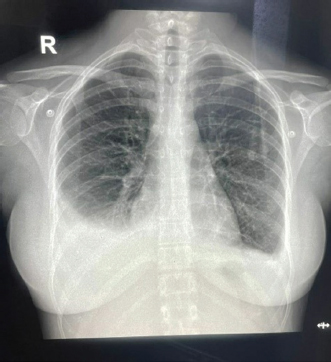
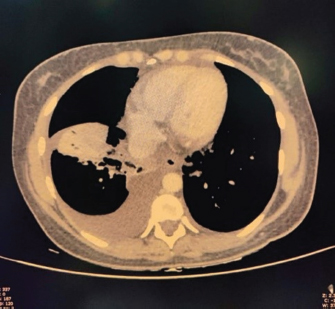
ABSTRACTBackground: Epstein-Barr virus (EBV) is a DNA virus that infects almost all adults. It is usually asymptomatic or causes a self-limiting illness but rarely can present serious complications. Case Description: We hereby report the case of a 28-year-old female who presented to us with a history of fever with malaise and an episode of Malena. She was hemodynamically stable. She had thrombocytopenia, elevated transaminases, and polyserositis on workup. She was evaluated for the same and found to be EBV positive with remaining infective and autoimmune workup within normal limits. Her symptoms persisted for a total duration of approximately 3 weeks before she became asymptomatic and was discharged. Conclusion: This case highlights that EBV should be considered as a differential diagnosis in such cases with atypical presentations especially in young adults. Keywords: Epstein-Barr virus, Infectious mononucleosis, Lymphadenopathy, Thrombocytopenia, Polyserositis. IntroductionEpstein-Barr virus (EBV) is a double-stranded DNA virus belonging to the Herpes virus family (Balows, 2023). It was discovered 32 years ago by Epstein, Achong, and Barr by using electron microscopy of cells cultured from Burkett’s lymphoma (Epstein et al., 1964). It is the causative agent of infectious mononucleosis which is a self-limiting illness. However, many complications can occur with EBV infection which are usually rare (Odumade et al., 2011). Through this case report, we highlight an unusual presentation of the EBV. Case DetailsA 28-year-old female, with a known case of hypothyroidism on regular treatment presented with high-grade fever associated with chills and rigors, burning micturition, generalized body ache, and headache for 9 days. She also had diffuse dull aching abdominal pain and a dry cough for 7 days. She had an episode of dark-colored stools 1 day back. On examination, she was conscious and oriented to time, place, and person. She had sinus bradycardia (Pulse rate–56/minute) with BP–120/80 in the right arm in the supine position, Spo2–96 % on room air, and was afebrile. On auscultation of the chest, she had reduced air entry in the bilateral infrascapular region with a dull note of percussion in these areas. The rest of the systemic examination was normal. On investigation, she was found to have anemia (Hb 10.4g/dl), raised TLC (13,000/cumm), thrombocytopenia (26,000/cumm), and elevated transaminases (SGOT/SGPT – 451/224 IU/l), with a normal coagulation profile.KFT was grossly normal (Table 1). Inflammatory workup revealed: ESR–22 mm Hg in 1 hour, CRP–53.2 mg/dl, Procalcitonin–1.92 ng/ml. D dimer–1.9 mg/l. Ferritin–2,500 ng/ml. Chest X-ray revealed blunting of bilateral costophrenic angles suggestive of bilateral pleural effusion (Fig. 1). USG’s whole abdomen revealed hepatomegaly and mild to moderate ascites. In view of melena and thrombocytopenia, 6 units of random donor platelets were transfused. A workup for fever with thrombocytopenia and polyserositis was started. Dengue RTPCR, NS1 antigen, IgM, Weil felix, and VDRL were negative. Viral markers (HIV/HBsAg/Anti HCV/Anti HAV IgM/Anti HEV IgM) were negative. Scrub IgM, Brucella IgM, Urinary legionella antigen, CMV PCR, and Leptospira IgM were negative. Malaria Ag and P/S for the malaria parasite were negative. The widal test and Typhidot IgM were negative. CPK and CK-MB were negative. Stool occult blood (three samples) were negative. Covid PCR was negative. Blood and urine C/S were negative. Urine KOH revealed few budding yeast cells. Urine fungal culture was negative. Workup for EBV revealed 24,300 copies of EBV RTPCR and EBV IgM and IgG were positive. We did an autoimmune workup considering a young female with polyserositis but both ANA by Immunofluorescence and ANA profile were negative. Direct and indirect Coombs tests were negative. By the end of first week of admission, thrombocytopenia started improving but she had a persistent cough and fever although spikes were reduced. A 2D echo revealed trace pericardial effusion. Repeat Chest X-ray revealed persistent bilateral pleural effusion (Right > Left) (Fig. 2). In view of persistent effusion, we decided to perform a CECT chest and abdomen which revealed evidence of moderate right pleural effusion, minimal fluid in the left pleural cavity with atelectatic changes in the adjacent lung, oedematous thickened gallbladder and mild ascites (Figs. 3 and 4). Table 1. Routine investigations.
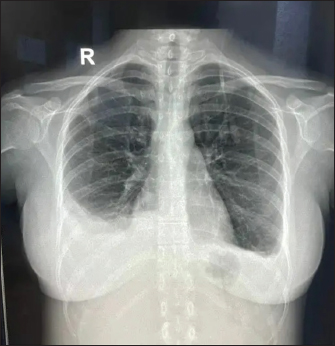
Fig. 1. X-ray showing blunting of bilateral costophrenic angles (Right > left) suggestive of pleural effusion. Under USG guidance the pleural fluid was aspirated and sent for examination which was grossly normal. Pleural fluid gram stain, fungal KOH smear, TB Xpert, and MGIT/Rapid TB culture were negative. Pleural fluid, total protein 2.84 gm/dl, albumin 1.77 gm/dl, LDH 275 U/l, cell counts were 265 (97% lymphocyte and 3% neutrophil), and ADA 15.39 U/l. ANA IF was negative in a pleural fluid sample. A final diagnosis of EBV-associated polyserositis, thrombocytopenia, and elevated transaminases was made. The patient was managed conservatively and fever subsided on Day 10 of admission. Repeat chest Xray showed resolving effusion. The patient was finally discharged after 10 days of admission with advice to follow up. On follow up, the effusion has completely resolved and the patient is doing absolutely fine.
Fig. 2. Repeat chest X-ray showing persistent bilateral pleural effusion (Right > Left).
Fig. 3. CECT chest suggestive of bilateral pleural effusion Right > left, minimal pericardial effusion.
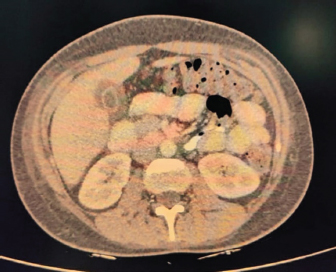
Fig. 4. CECT abdomen suggestive of edematous thickened gall bladder and mild ascites. DiscussionTo the best of our knowledge, this is one of the first few case reports of EBV-associated polyserositis. EBV is a double-stranded DNA virus that infects B lymphocyte cells. The virus causes B cells to differentiate into memory B cells, which then become latent until a trigger causes reactivation (Odumade et al., 2011). A high titer of viral DNA is found in the salivary secretions of the infected patients. Deep kissing and food sharing are therefore the important modes of transmission (Odumade et al., 2011). The transmission has also been reported through stem cells, organ transplantation, and blood transfusion rarely (Odumade et al., 2011; Dunmire et al., 2018). EBV has infected nearly 95 % of adults in the world (Womack and Jimenez, 2015). It can remain asymptomatic or can manifest as a complete spectrum of illnesses (Hoover and Higginbotham, 2023). Over 50 % of patients present as a clinical triad of fever, sore throat, and generalized lymphadenopathy termed as infectious mononucleosis (Andreoli et al., 2004). Infectious mononucleosis can occur in all age groups but most cases are reported in adolescence and early adulthood. EBV is predominantly a clinical diagnosis with supportive laboratory findings. The majority of patients have leucocytosis with an absolute increase in peripheral mononuclear cells and atypical T lymphocytes on peripheral smears (Epstein et al., 1964). Heterophile antibody tests can detect IgM antibodies to EBV but can result in false positive results (Womack and Jimenez, 2015). Viral capsid antigen (VCA) IgM and IgG can be used to confirm the diagnosis. IgM antibodies to VCA disappear in 4–6 weeks therefore their presence detects an acute infection B, while increased VCA IgG antibody levels indicate prior or chronic infection (Odumade et al., 2011). Infectious mononucleosis is a self-limiting disease with a relatively good prognosis as most patients improve with time with symptomatic treatment (Rea et al., 2001). Differential diagnoses for infectious mononucleosis include Acute viral/bacterial tonsillitis, Acute HIV, CMV, syphilis, and Toxoplasma gondii (Vincent et al., 2004). Complications occur rarely and depend on the immunopathologic response to the virus (Cohen, 1997), 10 % of patients present with splenomegaly, hepatomegaly, and palatal petechiae (Cohen, 2000). Another rare complication of infectious mononucleosis from EBV is airway obstruction from tonsillar edema of the pharyngeal tissues (Womack and Jimenez, 2015). There are many other complications from EBV infection that can occur such as myocarditis, encephalitis, hemophagocytic lymphohistiocytosis, pancreatitis, hepatitis, pleural, pericardial effusion and autoimmune hemolytic anemia (Watanabe et al., 2020). EBV has also been implicated in causing lymphomas and nasopharyngeal cancer (Odumade et al., 2011). Treatment of EBV is mainly symptomatic. Our patient was a young female with fever, myalgia, thrombocytopenia, elevated liver enzymes, and polyserositis. The patient being a resident of Delhi, we considered a differential diagnosis of dengue fever, malaria, and enteric fever. However, all were negative. Scrub Typhus and Leptospira were also negative. Considering her a young female with fever and myalgia, we did an autoimmune workup as well; however, it was also negative. Finally, the EBV came positive. Patient was managed symptomatically and her symptoms resolved over 3 weeks. ConclusionThis case highlights that EBV should be considered as a differential diagnosis in such cases with atypical presentations especially in young adults. ReferencesAndreoli, T., Carpanter, C., Griggs, R. and Loscalzo, J. 2004. Cecil essentials of medicine, 6th ed. Philadelphia, PA: WB Saunders, pp: 829–833. Balows, A. 2003. Manual of clinical microbiology. 8th edition: P. R. Murray, E. J. Baron, J. H. Jorgenson, M. A. Pfaller, and R. H. Yolken, eds., ASM Press. Diagn. Microbiol. Infect. Dis. 47(4), 625–626. Cohen, J.I. 1997. Epstein—Barr virus and the immune system: hide and seek. JAMA 278, 510–513. Cohen, J.I. 2000. Epstein—Barr virus infection. NEJM 343, 481–492. Dunmire, S.K., Verghese, P.S. and Balfour, H.H. 2018. Primary Epstein-Barr virus infection. J. Clin. Virol. 102, 84–92. Epstein, M.A., Achong, B.G. and Barr, Y.M. 1964. Virus particles in cultured lymphoblast from Burkett’s lymphoma. Lancet 1, 702–703. Hoover, K. and Higginbotham, K. 2023. Epstein-Barr virus [Updated 2022 Aug 8]. [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan. Available via https://www.ncbi.nlm.nih.gov/books/NBK559285/report=classic Odumade, O.A., Hogquist, K.A. and Balfour, H.H. 2011. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin. Microbiol. Rev. 24, 193–209. Rea, T.D., Russo, J.E., Katon, W., Ashley, R.L. and Buchwald, D.S. 2001. Prospective study of the natural history of infectious mononucleosis caused by Epstein-Barr virus. J. Am. Board Fam. Pract. 14, 234–242. Vincent, M.T., Celestin, N. and Hussain, A.N. 2004. Pharyngitis. Am Fam Physician. 69, 1465–1470. Watanabe, M., Panetta, G.L., Piccirillo, F., Spoto, S., Myers, J., Serino, F.M., Costantino, S. and Di Sciascio, G. 2020. Acute Epstein-Barr related myocarditis: an unusual but life-threatening disease in an immunocompetent patient. J. Cardiol. Cases. 21, 137–140. Womack, J. and Jimenez, M. 2015. Common questions about infectious mononucleosis. Am. Fam. Physician. 91, 372–376. | ||
| How to Cite this Article |
| Pubmed Style Kumar R, Khosla P, Taneja V, Dessai R, Sondhi M. Epstein-Barr virus-associated polyserositis, thrombocytopenia, and elevated transaminases in a young female. J Microbiol Infect Dis. 2024; 14(1): 30 -33 . doi:10.5455/JMID.2024.v14.i1.5 Web Style Kumar R, Khosla P, Taneja V, Dessai R, Sondhi M. Epstein-Barr virus-associated polyserositis, thrombocytopenia, and elevated transaminases in a young female. https://www.jmidonline.org/?mno=184596 [Access: January 25, 2026]. doi:10.5455/JMID.2024.v14.i1.5 AMA (American Medical Association) Style Kumar R, Khosla P, Taneja V, Dessai R, Sondhi M. Epstein-Barr virus-associated polyserositis, thrombocytopenia, and elevated transaminases in a young female. J Microbiol Infect Dis. 2024; 14(1): 30 -33 . doi:10.5455/JMID.2024.v14.i1.5 Vancouver/ICMJE Style Kumar R, Khosla P, Taneja V, Dessai R, Sondhi M. Epstein-Barr virus-associated polyserositis, thrombocytopenia, and elevated transaminases in a young female. J Microbiol Infect Dis. (2024), [cited January 25, 2026]; 14(1): 30 -33 . doi:10.5455/JMID.2024.v14.i1.5 Harvard Style Kumar, R., Khosla, . P., Taneja, . V., Dessai, . R. & Sondhi, . M. (2024) Epstein-Barr virus-associated polyserositis, thrombocytopenia, and elevated transaminases in a young female. J Microbiol Infect Dis, 14 (1), 30 -33 . doi:10.5455/JMID.2024.v14.i1.5 Turabian Style Kumar, Rahul, Pooja Khosla, Vinus Taneja, Rishikesh Dessai, and Manuj Sondhi. 2024. Epstein-Barr virus-associated polyserositis, thrombocytopenia, and elevated transaminases in a young female. Journal of Microbiology and Infectious Diseases, 14 (1), 30 -33 . doi:10.5455/JMID.2024.v14.i1.5 Chicago Style Kumar, Rahul, Pooja Khosla, Vinus Taneja, Rishikesh Dessai, and Manuj Sondhi. "Epstein-Barr virus-associated polyserositis, thrombocytopenia, and elevated transaminases in a young female." Journal of Microbiology and Infectious Diseases 14 (2024), 30 -33 . doi:10.5455/JMID.2024.v14.i1.5 MLA (The Modern Language Association) Style Kumar, Rahul, Pooja Khosla, Vinus Taneja, Rishikesh Dessai, and Manuj Sondhi. "Epstein-Barr virus-associated polyserositis, thrombocytopenia, and elevated transaminases in a young female." Journal of Microbiology and Infectious Diseases 14.1 (2024), 30 -33 . Print. doi:10.5455/JMID.2024.v14.i1.5 APA (American Psychological Association) Style Kumar, R., Khosla, . P., Taneja, . V., Dessai, . R. & Sondhi, . M. (2024) Epstein-Barr virus-associated polyserositis, thrombocytopenia, and elevated transaminases in a young female. Journal of Microbiology and Infectious Diseases, 14 (1), 30 -33 . doi:10.5455/JMID.2024.v14.i1.5 |