| Research Article | ||
J Microbiol Infect Dis. 2023; 13(4): 175-181 J. Microbiol. Infect. Dis., (2023), Vol. 13(4): 175–181 Original Research Prevalence and risk factors of methicillin resistance in small colony variant versus normal colony variant of Staphylococcus aureus and their clinical outcome at tertiary care referral center: A prospective cross-sectional studyAmresh Kumar Singh*, Nitin Kumar Singh, Ankur Kumar, and Sarita KumariDepartment of Microbiology, Baba Raghav Das Medical College, Gorakhpur, India *Corresponding Author: Amresh Kumar Singh. Department of Microbiology, Baba Raghav Das Medical College, Gorakhpur, India. Email: amesh.sgpgi [at] gmail.com Submitted: 24/07/2023 Accepted: 01/12/2023 Published: 31/12/2023 © 2023 Journal of Microbiology and Infectious Diseases
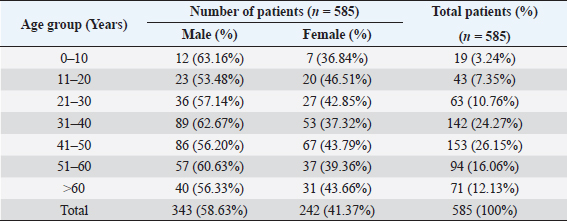
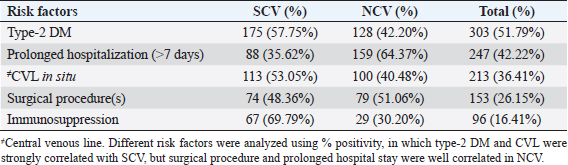
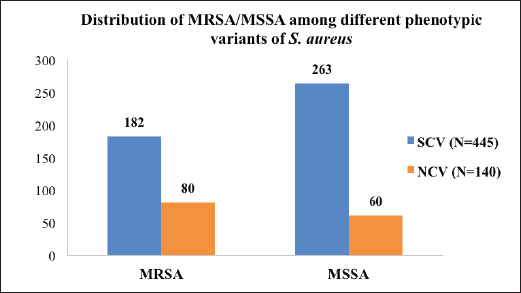
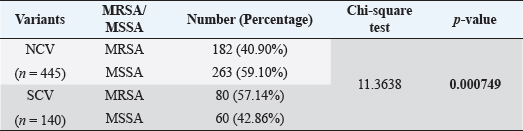
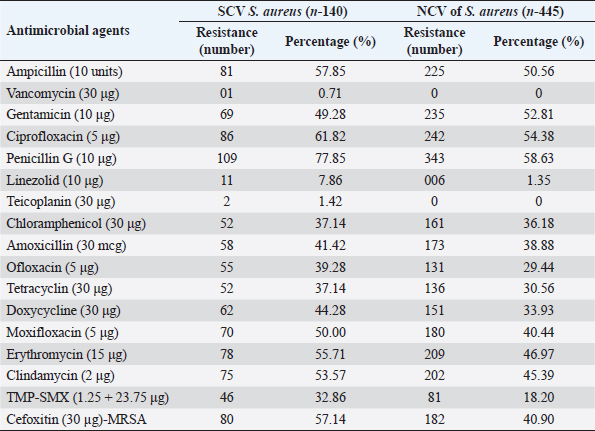
AbstractBackground: The ability of Staphylococcus aureus to maintain a clinically very indolent state depends on various lifestyles the bacteria evolved as small colony variants (SCVs) phenotype to safeguard themselves causes chronicity of the infection, providing resistance to the host challenge and antibiotics. Aim: A prospective study has been conducted on none-duplicated S. aureus isolates from bloodstream infections to assess the prevalence, risk factors, and clinical effect/outcome of SCV versus normal colony variant (NCV) patients. Methods: We conducted a prospective study considering the characteristics morphology, identification, and antimicrobial resistance patterns of SCV were also investigated as per standard guidelines (Clinical and Laboratory Standards Institute 2023). Results: A total of 585 positive blood culture samples were collected with the growth of S. aureus. Among which 140 (23.93%) isolates came out to be SCV, remaining 445 (76.07%) were NCV of S. aureus. The prevalence of methicillin resistance among S. aureus (MRSA) among 140 SCV came out to be 80 (57.14%) as compared with 445 NCV was 182 (40.90%). In this study majority with type-2 DM (51.79%), prolonged hospitalization (42.22%), and the placement of central line (36.41%). The mortality rate was 7.69%, in which the majority of the patients belong to NCV, (84.44%) followed by SCV (15.55%). Conclusion: The SCV phenotype and antibiotic resistance are interlinked; we still need to learn more about the clinical consequences of infection as well as the risk factors for SCV MRSA. These results highlight the importance of SCV infection and support the routine use of clinical laboratory tests that are sensitive to SCV. Keywords: Staphylococcus aureus, Small colony variants, Methicillin-resistant S. aureus, Multidrug-resistant bloodstream infections. IntroductionGram-positive bacteria Staphylococcus aureus is a common resident of the human skin and nasal microbiota in many people, but it also plays a significant role as the etiological agent of a number of serious infections, including those of the skin and soft tissue (Zheng et al., 2021). Staphylococcus aureus small-colony variant (SCV) is prevalent in infections that are resistant to antibiotic treatment, including osteomyelitis, chronic airway infections in people with cystic fibrosis, and infections brought on by medical devices. SCV of S. aureus is detected in antibiotic-resistant diseases such as osteomyelitis, chronic airway infections in cystic fibrosis patients, and device-related infections (Proctor, 2019). These naturally occurring variations benefit from the capacity to remain within eukaryotic cells, which shields them from host defenses and antibiotics. SCV have non-pigmented, non-hemolytic colonies that are 10 times smaller than the typical phenotypic. This small size is frequently caused by auxotrophy for specific growth factors such as menadione, hemin, and/or thymidine (Garcia et al., 2013). SCV are characterized phenotypically by a slow growth rate, aberrant colony form, and odd biochemical characteristics, making detection by clinical microbiology laboratories a major challenge. The metabolic abnormalities also affect their susceptibility to antibiotics, which, when paired with an ability to survive intracellular and, in the case of some strains, to form biofilms, makes them more resistant to antibiotics (Garcia et al., 2013). Because they require particular nutritional support and prolonged culture for isolation, their incidence and prevalence in clinical specimens may be greatly underestimated (von Eiff, 2008). In clinical isolates, two forms of SCV are found: electron-transport-defective strains that are auxotrophs for menadione or hemin, and thymidine auxotrophs (Kriegeskorte et al., 2011). SCV of S. aureus is a slow-growing bacterial subpopulation with different morphological and pathogenic characteristics. SCVs have a slow growth rate, abnormal colony form, and odd biochemical properties, making them difficult to diagnose for clinical microbiologists (Proctor et al., 2006). Since their discovery, SCVs have been described for a diverse range of bacterial genera and species, including S. aureus, meticillin (formerly methicillin)-resistant S. aureus, Staphylococcus epidermidis, Staphylococcus capitis, Pseudomonas aeruginosa, Burkholderia cepacia, Salmonella enterica, Vibrio cholerae, Escherichia coli, and Neisseria gonorrheae. SCV from other genera and species have also been recovered from clinical specimens, including abscesses, blood bones and joints, the respiratory tract, and soft tissues (Proctor et al., 2006). There is growing evidence that S. aureus infections, particularly those that are persistent, relapsing, and difficult to treat, are related to the establishment of the SCV phenotype (Gläser et al., 2014). SCV of S. aureus that changed in relevant phenotype have made therapeutic options against S. aureus infections increasingly limited and difficult. Rifampicin is classified as the “last-resort” antibiotic against S. aureus (Zheng et al., 2021). When S. aureus is internalized in the host cell milieu, it can transform from the wild-type phenotype to the SCV phenotype, allowing it to evade host defensive responses and spread the infection (Tuchscherr et al., 2011). Multidrug-resistant bloodstream infections (BSI) are a major cause of death and morbidity, particularly in pediatric populations. Bacteremia affects around 200,000 people each year, with fatality rates ranging from 20% to 50% (Singh et al., 2019). The objective of this study was to determine the frequency of S. aureus isolated from blood stream infection patients, as well as their antimicrobial resistance pattern, in order to determine the current prevalence, risk factors, and outcome of different colony variants of methicillin resistance among S. aureus (MRSA) isolated from BSIs in a tertiary care teaching hospital. Materials and MethodsSpecimenA prospective study was conducted at Department of Microbiology, BRD Medical College and associated Nehru Hospital Gorakhpur UP, Bharat over a period of 1 year from January to December 2022. In 1 year, study period, 585 culture positive blood samples of S. aureus were collected from clinically suspected septicemia patients admitted to the hospital. Inclusion criteriaPatients with a clear diagnosis of BSI existed at the time of admission or occurred more than 48 hours after admission with confirm case of BSI caused by S. aureus. Exclusion criteriaAll outdoor patients, non-BSI/sterile blood culture, coagulase negative Staphylococcus, left against medical advice patients were excluded. Patient demographyAll recruited study subject demographic data has been entered in software includes age, sex, day of testing, day of culture positivity, types of organism/colony variants, different risk factors (Type-2 DM, prolonged hospitalization, central line, immunosuppression, etc.), and co-resistance with other antibiotics in SCV and normal colony variant (NCV). Blood culture and micro-organism identificationBlood samples were obtained by trained health professionals using conventional aseptic protocols, immediately transported to the Department of Microbiology, and aerobically inoculated at 37°C into brain heart infusion broth (HiMedia, Mumbai, India). Subcultures were performed on the third, fifth, and seventh days to fresh 5% sheep blood agar, MacConkey’s agar, and nutrient agar for bacterial colony characteristics (SCV vs. NCV). The growth requirements of SCVs are very particular. The staphylococcal phenotype is typically characterized by medium colonies that develop into 1- to 3-mm diameter colonies in a matter of hours. On the other hand, SCV colonies are much smaller, about 1/10 the size of the original strain, and they resemble pinpoint colonies (Becker, 2023). For declaring a negative result, the broth was examined daily for signs of bacterial growth (turbidity, hemolysis, clot formation), and a final subculture was performed at the end of the seventh day. Second blood cultures were taken wherever they were required and processed similarly as per protocol. Gram staining, colony features, and biochemical testing (catalase test, coagulase test, and growth on selective media- mannitol salt agar) were used to detect positive growth (Jasuja et al., 2017). Antibiotic susceptibility testingThe antibiotic susceptibility testing of the isolates was examined using the Modified Kirby Bauer disc diffusion technique on Muller Hinton Agar according to the standard Clinical and Laboratory Standards Institute (CLSI, 2023) recommendations using commercially available antibiotic discs from Hi-Media (Mumbai, India) (CLSI, 2023). The following antibiotic disk were used- ampicillin (10 units), vancomycin (30 μg), gentamicin (10 μg), ciprofloxacin (5 μg), Penicillin G (10 μg), linezolid (10 μg), erythromycin (15 μg), clindamycin (20 μg), teicoplanin (30 μg), chloramphenicol (30 μg), amoxicillin (30 μg), ofloxacin (5 μg), tetracycline (30 μg), doxycycline (30 μg), moxifloxacin (5 μg), trimethoprim-sulfamethoxazole (TMP/SMX) (1.25 + 23.75 μg), and cefoxitin (30 μg) for MRSA. The zone of inhibition was determined after incubation of 24 hours at 37°C using CLSI 2023 guidelines. Statistical analysisAll the data were entered in Statistical Package for Social Sciences (SPSS) software version 22 (SPSS Inc. Chicago, IL) and analyzed accordingly in percentage, chi-square test, and one-way analysis of variance was used to determine significant differences at p < 0.05. Ethical approvalEthical approval was obtained from Institutional Ethical Committee before the beginning of the study. All the participants are informed consent to participate in this study with written informed consent. ResultsIn this study, a total of 585 nonduplicate isolates were enrolled for analysis. Deferent demographic parameters, types of colony variants, day of positivity, risk factors, and resistance pattern were analyzed to know the effect and outcome of both colony variants of S. aureus. In this study, the majority of patients (64.27%) come from rural areas. The rest 209 patients (35.72%) of all obtained specimens are from the urban region. In this study, out of 585 S. aureus isolates tested, 140 (23.93%) of the positive for SCV, whereas the remaining 445 (76.07%) tested positive for NCV. The bulk of these patients (58.63%) were males. In terms of age, the majority of patients are in their fifth decade of life, followed by their fourth, and finally their sixth (Table 1). In this investigation, 585 positive blood culture samples were obtained, all of which showed S. aureus growth. Out of these 585 samples, 364 (62.22%) were positive on the third day of incubation, while the remaining 221 (37.77%) were positive on the seventh day of incubation. In this study, the majority of patients (51.79%) had a history of type-2 diabetes, followed by prolonged hospitalization (42.22%), insertion of a central venous line (36.41%), undergone any major surgery (26.15%), and had a condition leading to immunosuppression (16.41%). In terms of risk factor associated with NCV or SCV, larger percentage of SCV-isolated patients were shown to be associated with many risk factors (Table 2). In this study, the majority of MRSA isolates (57.14%) were detected in SCV, but in NCV MRSA was (40.90%) (Fig. 1). MRSA was found in a statistically significant proportion of SCV of S. aureus as compared to NCV (Table 3). The antimicrobial susceptibility pattern of the SCV isolates of S. aureus showed maximum resistance to Penicillin G (77.85%) followed by ciprofloxacin (61.82%), and ampicillin (57.85%), while most of the isolates were sensitive for vancomycin, linezolid, and teicoplanin (Table 4) as compared to NCV where lesser number of isolates were resistant. Table 1. Showing demographic distribution (%) of patients with S. aureus infection.
Table 2. Showing association of different risk factors among SCV and NCV isolates of S. aureus (%).
Fig. 1. Distribution of MRSA/MSSA among different phenotypic variants (SCV vs. NCV) of S. aureus (number). Table 3. Showing MRSA/MSSA distribution (%) among the isolates and statistical difference (p value).
In this study, the mortality rate was 7.69%, in which the majority of the patients belong to NCV of S. aureus, (84.44%) followed by SCV of S. aureus (15.55%). DiscussionSCV of S. aureus usually causes chronic and recurring infections, although they are often overlooked, and there are only a few studies on these clinical isolates. The purpose of this study was to look at the prevalence and characteristics of S. aureus SCV (Min et al., 2022). SCV of S. aureus is known to contribute to different chronic infections, difficult to treat in different clinical settings (Suwantarat et al., 2018). In this study, SCV of S. aureus prevalence is 23.93% for all type of patients. A study carried out in 2019 shows the prevalence of SCV of S. aureus to 28% of all S. aureus isolated from cystic fibrosis patients which is concordant with this study (Wolter et al., 2019). Another study revealed that SCV of S. aureus to be 14%, which is non-concordant with this study (Suwantarat et al., 2018). In this study, most of the patients were from rural area. A study reported that the majority of the patients lived in urban area (77.1%) (Aliyu et al., 2018; Tsige et al., 2020). This is explained by the location of the hospital. The study findings show that the most common risk factor associated with getting S. aureus infection is having a history of type-2 DM, followed by prolonged hospitalization, followed by placement of the central venous line, followed any surgery and last is immunosuppression. Studies carried out in 2018 and 2019 show a similar type of association with risk factors (Aliyu et al., 2018; Liu et al., 2021), and the association of risk factors was not consistent with the study conducted by Mehl et al. (2017). The findings of this study demonstrated that SCV of MRSA appears to account for a significant proportion of MRSA. Notably, this study suggests that past antibiotic use may be a risk factor for SCV of MRSA infection. Furthermore, SCV of MRSA was found to be resistant to TMP/SMX (32.86%), an oral antibiotic commonly used to treat chronic MRSA infection. These findings are consistent with previously published data indicating that TMP/SMX exposure can produce the SCV phenotype and that the SCV of S. aureus has a higher resistance to this antibiotic (Kriegeskorte et al., 2015). This study also showed that SCV of MRSA is susceptible to long-established anti-MRSA medications such as vancomycin (0.17%). It should be noted that the results of this study suggest that SCV of MRSA is harder to treat with common antibiotics because of the re-selection of mutant SCV of S. aureus as compared to NCV of MRSA, especially in the context of pulmonary infection because vancomycin has relatively poor lung tissue penetration. Table 4. Showing antibiotic resistance patterns (number and %) among S. aureus strains.
In this study SCV of MRSA was about 57.14% which is quite higher side as compared to MRSA in NCV of S. aureus 40.89%, these results were concordant with a study done in 2019 as they stated MRSA prevalence among SCV is to be 56% (Suwantarat et al., 2018). Another study showed MRSA prevalence in NCV of S. aureus is to be 28.3% (Tsige et al., 2020), which is also lower than this study that shows the results are non-concordant with this study. The resistance pattern of the isolates in this study, the most resistant drugs were Penicillin G followed by ciprofloxacin and other common antibiotics suggested for S. aureus infections. The resistance to Penicillin G in this study is 77.85% which is concordant with the study done by DebreMarkos referral Hospital 82.2% (Kahsay et al., 2014). In other studies, Penicillin resistance was reported as 97% and 100% in Tanzania (Mshana et al., 2009) and Jimma, Ethiopia, respectively (Tsige et al., 2020). In the current investigation, resistance to clindamycin is comparable. While antibiotics are being used to treat persistent MRSA infections, this study offers evidence for a novel strategy to treat SCV of MRSA infection in MRSA that needs more investigation. Comparison between SCV of MRSA and NCV of MRSA are fundamentally limited by the fact that revertants between SCV and NCV of S. aureus do occur in many cases, making it difficult to accurately categorize patients into the SCV group vs. the NCV group (Suwantarat et al., 2018). Individuals who have co-infection of both NCV and SCVs are more likely to see revertants, according to one observation. These findings showed that SCV of MRSA may be strongly correlated with different risk factors and outcomes; however, a prospective, multicentric longitudinal study will be required to assess the existence of revertants and account for this phenomenon in the assessment of SCV MRSA’s clinical importance. ConclusionSCVs of S. aureus grow slowly, and their colony morphology and biochemical characteristics are significantly different from the classic/normal variant. SCV of MRSA accounted for a considerable proportion of MRSA infection, and SCV of MRSA patients were almost all chronically infected with MRSA. Antibiotic resistance is more connected with the SCV phenotype, and previous antibiotic use may be a risk factor for the emergence of SCV of MRSA. More research is needed to better understand the risk factors for SCV of MRSA and the clinical effects of infection. However, the underline mechanism and pathogenic characteristics of SCVs still need further studies. Most clinical laboratories do not regularly identify these variations. These findings emphasize the significance of the SCV infection and support the routine use of clinical laboratory tests that are sensitive for SCV identification and that distinguish SCV subtypes. AcknowledgmentsThe authors thank the editors and the anonymous reviewers for their insightful suggestions on this study. Conflict of interestThe authors declare no potential conflicts of interest concerning this article’s research, authorship, and/or publication. FundingNone. ReferencesAliyu, S., Cohen, B., Liu, J. and Larson, E. 2018. Prevalence and risk factors for bloodstream infection present on hospital admission. J. Infect. Prev. 19(1), 37–42. Becker, K. 2023. Detection, identification and diagnostic characterization of the Staphylococcal small colony-variant (SCV) phenotype. Antibiotics 12(9), 1446; https://doi.org/10.3390/antibiotics12091446. CLSI. 2023. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100, 30th ed. Wayne, PA: Clinical and Laboratory Standards Institute (33rd Edition). M100CLSI, 94-105. Available via https://clsi.org/ast-2023/ Garcia, L.G., Lemaire, S., Kahl, B.C., Becker, K., Proctor, R.A., Denis, O., Tulkens, P.M. and Van Bambeke, F. 2013. Antibiotic activity against small-colony variants of Staphylococcus aureus: review of in vitro, animal and clinical data. J. Antimicrob. Chemother. 68(7), 1455–1464. Gläser, R., Becker, K., Von Eiff, C., Meyer-Hoffert, U. and Harder, J. 2014. Decreased susceptibility of Staphylococcus aureus small-colony variants toward human antimicrobial peptides. J. Invest. Dermatol. 134(9), 2347–2350. Jasuja, K., Singh, A.K., Kumar, A., Jayesh, P., Upadhyay, V. and Gaur, V. 2017. Emergence of multi-drug resistant bacterial isolates causing bloodstream infections in paediatric patients at a tertiary care hospital. Br. J. Pharm. Med. Sci. 2(2), 398–407. Available via https://www.bjpmr.org/2020/01/15/issues-2-march-april-2017/ Kahsay, A., Mihret, A., Abebe, T. and Andualem, T. 2014. Isolation and antimicrobial susceptibility pattern of Staphylococcus aureus in patients with surgical site infection at Debre Markos Referral Hospital, Amhara Region, Ethiopia. Arch. Public. Health. 72(1), 16. Kriegeskorte, A., König, S., Sander, G., Pirkl, A., Mahabir, E., Proctor, R.A., von Eiff, C., Peters, G. and Becker, K. 2011. Small colony variants of Staphylococcus aureus reveal distinct protein profiles. Proteomics 11(12), 2476–2490. Kriegeskorte, A., Lorè, N.I., Bragonzi, A., Riva, C., Kelkenberg, M., Becker, K., Proctor, R.A., Peters, G. and Kahl, B.C. 2015. Thymidine-dependent Staphylococcus aureus small-colony variants are induced by trimethoprim-sulfamethoxazole [SXT] and have increased fitness during SXT challenge. Antimicrob. Agents. Chemother. 59(12), 7265–7272. Liu, Y., Cui, B.C., Pi, C.M., Yu, X.H., Liu, Z.W., Li, X., Ma, L.P. and Wang, C. 2021. Analysis of prognostic risk factors of bloodstream infections in Beijing communities: a retrospective study from 2015 to 2019. Mediterr. J. Hematol. Infect. Dis. 13(1), 1–7. Mehl, A., Åsvold, B.O., Kümmel, A., Lydersen, S., Paulsen, J., Haugan, I., Solligård, E., Damås, J.K., Harthug, S. and Edna, T.H. 2017. Trends in antimicrobial resistance and empiric antibiotic therapy of bloodstream infections at a general hospital in Mid-Norway: a prospective observational study. BMC. Infect. Dis. 17(1), 116; https://doi.org/10.1186/s12879-017-2210-6 Min, C., Wang, H., Xia, F., Tang, M., Li, J., Hu, Y., Dou, Q. and Zou, M. 2022. Characteristics of Staphylococcus aureus small colony variants isolated from wound specimen of a tertiary care hospital in China. J. Clin. Lab. Anal. 36(1), 1–9. Mshana, S., Kamugisha, E., Mirambo, M., Chalya, P., Rambau, P., Mahalu, W. and Lyamuya, E. 2009. Prevalence of clindamycin inducible resistance among methicillin resistant Staphylococcus aureus at Bugando Medical Centre, Mwanza, Tanzania. Tanzan. J. Heal. Res. 11(2), 59–64. Proctor, R. 2019. Respiration and small colony variants of Staphylococcus aureus. Microbiol. Spectr. 31, 7(3). Proctor, R.A., von Eiff, C., Kahl, B.C., Becker, K., McNamara, P., Herrmann, M. and Peters, G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4(4), 295–305. Singh, A.K., Tripathi, P., Kumar, A., Pandey, J. and Gaur, V. 2019. Role and efficacy of vancomycin-ceftriaxone combination for the treatment of serious Gram-positive bacterial infections at tertiary care centre. Int. J. Biomed. Adv. Res. 10(8), e5240; https://doi.org/10.7439/ijbar.v10i8.5240:e5240. Suwantarat, N., Rubin, M., Bryan, L., Tekle, T., Boyle, M.P., Carroll, K.C. and Jennings, M.T. 2018. Frequency of small-colony variants and antimicrobial susceptibility of methicillin-resistant Staphylococcus aureus in cystic fibrosis patients. Diagn. Microbiol. Infect. Dis. 90(4), 296–299; https://doi.org/10.1016/j.diagmicrobio.2017.11.012 Tsige, Y., Tadesse, S., Tefera, M.M., Amsalu, A., Menberu, M.A. and Gelaw, B. 2020. Prevalence of methicillin-resistant Staphylococcus aureus and associated risk factors among patients with wound infection at Referral Hospital, Northeast Ethiopia. J. Pathog. 2020, 1–7; https://doi.org/10.1155/2020/3168325 Tuchscherr, L., Medina, E., Hussain, M., Völker, W., Heitmann, V., Niemann, S., Holzinger, D., Roth, J., Proctor, R.A., Becker, K., Peters, G. and Löffler, B. 2011. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO. Mol. Med. 3(3), 129–141. von Eiff, C. 2008. Staphylococcus aureus small colony variants: a challenge to microbiologists and clinicians. Int. J. Antimicrob. Agents. 31(6), 507–510. Wolter, D.J., Onchiri, F.M., Emerson, J., Precit, M.R., Lee, M., McNamara, S., Nay, L., Blackledge, M., Uluer, A., Orenstein, D.M., Mann, M., Hoover, W., Gibson, R.L., Burns, J.L. and Hoffman, L.R. 2019. Prevalence and clinical associations of Staphylococcus aureus small-colony variant respiratory infection in children with cystic fibrosis [SCVSA]: a multicentre, observational study. Lancet. Respir. Med. 7(12), 1027–1038. Zheng, X., Fang, R., Wang, C., Tian, X., Lin, J., Zeng, W., Zhou, T. and Xu, C. 2021. Resistance profiles and biological characteristics of rifampicin-resistant Staphylococcus aureus small-colony variants. Infect. Drug. Resist. 14, 1527–1536. | ||
| How to Cite this Article |
| Pubmed Style Singh AK, Singh NK, Kumar A, Kumari S. Prevalence and risk factors of methicillin resistance in small colony variant vs. normal colony variant of Staphylococcus aureus and their clinical outcome at tertiary care referral center: A prospective cross-sectional study. J Microbiol Infect Dis. 2023; 13(4): 175-181. doi:10.5455/JMID.2023.v13.i4.2 Web Style Singh AK, Singh NK, Kumar A, Kumari S. Prevalence and risk factors of methicillin resistance in small colony variant vs. normal colony variant of Staphylococcus aureus and their clinical outcome at tertiary care referral center: A prospective cross-sectional study. https://www.jmidonline.org/?mno=158571 [Access: April 27, 2024]. doi:10.5455/JMID.2023.v13.i4.2 AMA (American Medical Association) Style Singh AK, Singh NK, Kumar A, Kumari S. Prevalence and risk factors of methicillin resistance in small colony variant vs. normal colony variant of Staphylococcus aureus and their clinical outcome at tertiary care referral center: A prospective cross-sectional study. J Microbiol Infect Dis. 2023; 13(4): 175-181. doi:10.5455/JMID.2023.v13.i4.2 Vancouver/ICMJE Style Singh AK, Singh NK, Kumar A, Kumari S. Prevalence and risk factors of methicillin resistance in small colony variant vs. normal colony variant of Staphylococcus aureus and their clinical outcome at tertiary care referral center: A prospective cross-sectional study. J Microbiol Infect Dis. (2023), [cited April 27, 2024]; 13(4): 175-181. doi:10.5455/JMID.2023.v13.i4.2 Harvard Style Singh, A. K., Singh, . N. K., Kumar, . A. & Kumari, . S. (2023) Prevalence and risk factors of methicillin resistance in small colony variant vs. normal colony variant of Staphylococcus aureus and their clinical outcome at tertiary care referral center: A prospective cross-sectional study. J Microbiol Infect Dis, 13 (4), 175-181. doi:10.5455/JMID.2023.v13.i4.2 Turabian Style Singh, Amresh Kumar, Nitin Kumar Singh, Ankur Kumar, and Sarita Kumari. 2023. Prevalence and risk factors of methicillin resistance in small colony variant vs. normal colony variant of Staphylococcus aureus and their clinical outcome at tertiary care referral center: A prospective cross-sectional study. Journal of Microbiology and Infectious Diseases, 13 (4), 175-181. doi:10.5455/JMID.2023.v13.i4.2 Chicago Style Singh, Amresh Kumar, Nitin Kumar Singh, Ankur Kumar, and Sarita Kumari. "Prevalence and risk factors of methicillin resistance in small colony variant vs. normal colony variant of Staphylococcus aureus and their clinical outcome at tertiary care referral center: A prospective cross-sectional study." Journal of Microbiology and Infectious Diseases 13 (2023), 175-181. doi:10.5455/JMID.2023.v13.i4.2 MLA (The Modern Language Association) Style Singh, Amresh Kumar, Nitin Kumar Singh, Ankur Kumar, and Sarita Kumari. "Prevalence and risk factors of methicillin resistance in small colony variant vs. normal colony variant of Staphylococcus aureus and their clinical outcome at tertiary care referral center: A prospective cross-sectional study." Journal of Microbiology and Infectious Diseases 13.4 (2023), 175-181. Print. doi:10.5455/JMID.2023.v13.i4.2 APA (American Psychological Association) Style Singh, A. K., Singh, . N. K., Kumar, . A. & Kumari, . S. (2023) Prevalence and risk factors of methicillin resistance in small colony variant vs. normal colony variant of Staphylococcus aureus and their clinical outcome at tertiary care referral center: A prospective cross-sectional study. Journal of Microbiology and Infectious Diseases, 13 (4), 175-181. doi:10.5455/JMID.2023.v13.i4.2 |