| Review Article | ||
J Microbiol Infect Dis. 2024; 14(3): 95-102 J. Microbiol. Infect. Dis., (2024), Vol. 14(3): 95–102 Review Article Chicken immunoglobulin as an alternative treatment for bacterial infections, emphasizing advantages, disadvantages, and mechanismsZahra Esmaeili1,2, Sara Kamal Shahsavar1,2, Masoud Keikha3 and Kiarash Ghazvini1,2*1Department of Microbiology and Virology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran 2Antimicrobial Resistance Research Center, Mashhad University of Medical Sciences, Mashhad, Iran 3Department of Nursing, School of Nursing and Midwifery, Iranshahr University of Medical Sciences, Iranshahr, Iran *Corresponding Author: Kiarash Ghazvini. Department of Microbiology and Virology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. Email: GhazviniK [at] mums.ac.ir Submitted: 22/06/2024 Accepted: 19/08/2024, Published: 30/09/2024 © 2024 Journal of Microbiology and Infectious Diseases
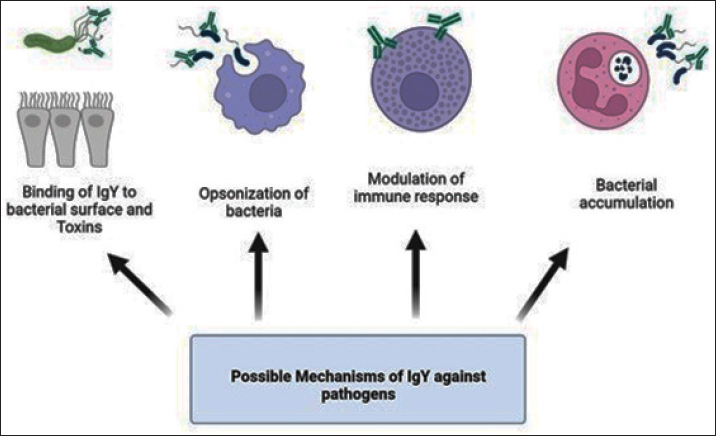
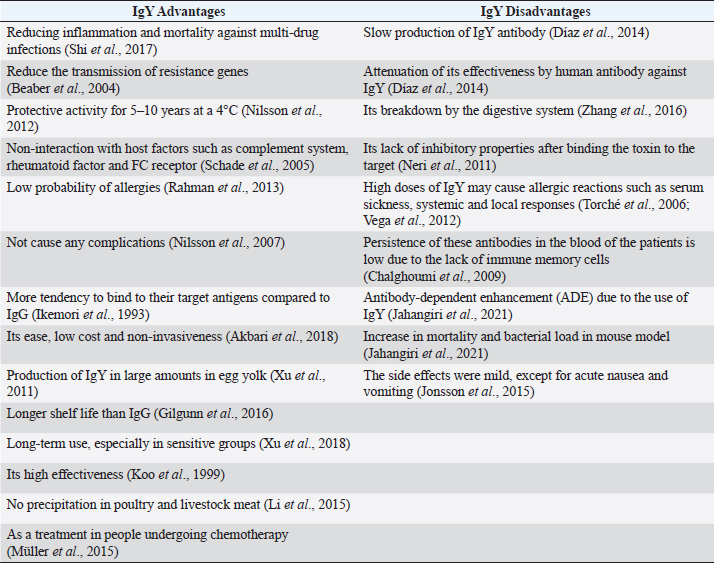
ABSTRACTThe overuse of antibiotics has led to an alarming spread of drug-resistant microbial infections, creating an urgent need for new therapeutic technologies. This issue has become a significant concern in recent years due to the increase in mortality rates, especially in hospital infections. In addition to antibiotic resistance, side effects caused by antibiotics, such as liver and kidney complications, threaten immunocompromised patients, infants, and the elderly, which indicates the need for immediate action. One of these technologies that has attracted attention as an alternative or complementary treatment for bacterial and viral infections is chicken immunoglobulin (IgY). This process involves extracting chicken immunoglobulin antibodies from egg yolk, which is achieved by injecting killed or recombinant pathogen antigens into the chicken. Several studies have investigated the therapeutic effects of IgY on bacterial infections in vitro and in vivo. However, a research gap exists regarding the mechanism of action, benefits, and possible side effects of these antibodies. This review article examines the structure, mechanism of action, optimal production conditions, advantages, and disadvantages of using this antibody, which can be widely used in the future. Keywords: Adverse reactions, Antibody, Immunoproteins, Infection control, Therapeutic effects. IntroductionOver a century ago, the initial proof of IgY production and transfer from a mother to an egg yolk was discovered. This type of immunity can safeguard the fetus for 2 weeks. It was first considered for human use in 1999 (Klemperer, 1893; Hamal, et al., 2006; Gadde et al., 2015). IgY is an antibody found in egg yolk that can be passed from mother to fetus, similar to IgG in humans (Sunwoo et al., 2002), and found not only in birds but also in reptiles, amphibians, and even fish. Its structure is similar to that of human IgG in terms of Fab and Fc fragments and function. It can be detected in large quantities, up to 100 mg per egg yolk (Warr et al., 1995; Schade et al., 2005). Despite their structural, functional, and frequency similarities, studies have shown a closer evolutionary relationship between IgY and IgE than between IgY and IgG (Taylor et al., 2009). This antibody can maintain stability in a pH range from 4 to 9 and at temperatures above 65°C. It also remains stable in the presence of pepsin at the mentioned pH, ensuring its protective effect is preserved. This allows it to be stored in powder form for several months, even outside the refrigerator (Wen et al., 2012; Thu et al., 2017). IgY structureBirds have antibodies similar to humans, such as IgA and IgM, but they also have another type of antibody called IgY. It is considered the most dominant type of antibody. Its structure is similar to mammalian IgG but lacks a hinge region. This structural difference results in a much longer half-life than IgG (Rose et al., 1974; Pereira et al., 2019). IgG and IgY are two types of antibodies that have structural differences. One of the differences is that IgY has a variable region and four constant regions, while IgG has fewer constant regions. IgY is larger with a molecular weight of 167 kDa, consisting of two heavy chains of 65 kDa and two light chains of 19 kDa. On the other hand, IgG has a smaller Fc region, making it less hydrophobic, and has a higher isoelectric pH than IgY. Finally, the molecular weight of IgY is approximately seven kDa greater than IgG’s (Sun et al., 2001). Under non-reducing conditions, its molecular weight can increase from 167 kDa to 180 kDa (Leiva et al., 2019). IgY mechanismsThere are multiple hypotheses for this case. One method to prevent bacteria from binding to host cell surface receptors is to inhibit their interaction (Lee et al., 2002). On the other hand, the binding of IgY to bacterial surface components such as pili, flagellum, and Omps may disrupt their activity and have a reducing effect on Quorum sensing and cellular signaling (Xu et al., 2011). Due to the importance of other neutralizing antibodies, IgY may play a role in neutralizing toxins and infections through this pathway. (Nilsson et al., 2007a,b). A study on the effect of IgY in Salmonella showed that this antibody can balance the production of inflammatory cytokines such as IFN-γ and TNF-α and modulate the immune response (Li et al., 2016). One of the direct impacts of IgY is its ability to significantly decrease bacterial growth, thereby effectively reducing pathogen growth in a dose-dependent manner (Schwartz et al., 2022). PMN cells play a crucial role in the innate immune system by controlling primary infections and inflammation (Thomsen et al., 2015). Opsonization of bacteria can be increased by factors such as IgG and complement (Joiner et al., 1984). Previous studies have demonstrated its role in phagocytic activity. It can induce polymorphonuclear (PMN) cells through physicochemical changes in bacteria, such as increased hydrophobicity and bacterial accumulation (Keller and Stiehm, 2000; Thomsen et al., 2015). This increase in phagocytosis by PMN cells may be due to changes in the electric charge of the bacterial surface and their interaction (Lee et al., 2002). The accumulation and clamping can change the geometry and structure of the pathogen, leading to bacterial immobilization and phagocytosis by immune cells (Champion and Mitragotri, 2006). Electron microscope investigations were conducted to study the effect of IgY on multi-drug-resistant Acinetobacter strains. The results showed that this antibody can inhibit Acinetobacter by increasing bacterial agglutination (Tsubokura et al., 1997). These findings have the potential to significantly enhance the rate of removal of pathogenic bacteria by altering the saturation of PMNs (Carlander et al., 1999a,b). In general, mechanisms such as inhibiting bacterial attachment, increasing opsonization of pathogens by PMs, modulating immunity, and finally neutralizing toxins are more prominent. The various hypotheses mentioned above are given in Figure (1). Optimal production and purification of IgYDifferent types of antigens, such as complex (virus and bacteria) and single (protein, nucleic acid, and polysaccharide) antigens, have been used to produce specific IgY in birds (Pereira et al., 2019). Different concentrations of antigens can be combined with various adjuvants that differ in chemical properties and immune system stimulation ability (Schade et al., 2005; Kovacs-Nolan and Mine, 2012; Savoldi et al., 2018; Pereira et al., 2019). While Freund’s complete adjuvant (FCA) is a very effective adjuvant for antibody production, it has some risks. It is known to cause severe inflammation at the injection site, which can lead to severe reactions. Unlike FCA, FIA does not contain mycobacteria and is less potent but causes fewer side effects. Therefore, it is better to use FCA for the first inoculation and switch to FIA for subsequent inoculations to minimize inflammation at the injection site (Schade et al., 2005; Kovacs-Nolan and Mine, 2012; Pereira et al., 2019). After producing the desired antigen, which can be either the complete cell form or a recombinant antigen with adjuvant, it is injected intramuscularly into the breast muscle of 6-month-old chickens. After 3 weeks of immunization, the eggs are collected. The number of required inoculations varies based on the type and dose of antigen, as well as the adjuvant used (Pereira et al., 2019). If the antibody titer decreases, more vaccinations should be administered during the spawning period to boost the antibody titer (Pereira et al., 2019). Therefore, the success of a successful immunization depends on several variables, including the intervals between the first, second, and subsequent vaccinations (Schade et al., 2005). One pivotal factor in optimizing the production of IgY is the breed of chicken used for immunization. For instance, the Rhode Island Red chicken breed demonstrates its potential by producing almost twice the amount of antibodies as the Single Comb White Leghorns. This highlights the significant impact of breed selection on immunization outcomes (Amro et al., 2018). Long light periods can reduce acquired immunity and antibody production in chickens. Providing 6 hours of darkness daily can improve antibody production (Hofmann et al., 2020). Recent studies have compared different methods such as water dilution, PEG, caprylic acid, chloroform, phenol, and carrageenan for the extraction and purification of IgY. The practical implications of these findings are significant. This research shows that water, PEG, and carrageenan dilution methods provide the highest yield, purity, and lipid residue, respectively. On the other hand, the chloroform method provides the highest filtration rate and protein concentration. Although water and PEG dilutions are the most commonly used methods, chloroform may be preferred in some cases (Ren et al., 2016; Shikun Ge, 2020). The isolation of IgY antibodies can be achieved through the polyethylene glycol 6,000 precipitation method from egg yolk. The extracted antibodies can be further purified through chromatography using the SDS page and western blotting before it is confirmed (Amro et al., 2018). One of the significant challenges in preparing IgY is purification and separation from yolk lipoproteins. As a first step, the water-soluble part containing IgY is separated from the lipid part (Kovacs-Nolan and Mine 2012). Various techniques have been suggested for isolating yolk lipoproteins. These methods include the precipitation of lipoprotein by polyethylene glycol and dextran sulfate, purification by organic solvents, dilution of yolk, and centrifugation at high speeds, ultrafiltration, and usage of natural polysaccharides such as xanthan gum, carrageenan, and sodium alginate (Mine and Kovacs-Nolan 2002; Kovacs-Nolan and Mine, 2012; Siriya et al., 2013; Pereira et al., 2019). To store this antibody for a longer duration, it can be kept in a refrigerator at 4°C for up to 5 years. However, previous studies have shown that the half-life of IgY in the gastrointestinal tract is only 1.73 hours. Therefore, it is necessary to use delivery methods like liposomes to increase the antibody’s efficiency (Yokoyama et al., 1993; Rahman et al., 2013; Li et al., 2022). IgY advantagesAntibiotic resistance, particularly in hospital infections, has become a severe threat. For instance, some studies have reported multidrug resistance in Acinetobacter baumannii strains up to 74% (Pourhajibagher et al., 2016). IgY can potentially treat infections by reducing inflammation and mortality in vitro and in vivo against multi-drug infections (Shi et al., 2017). One of the benefits of antibodies is that they cause less SOS response in bacteria, reducing the transmission of resistance genes (Beaber et al., 2004). Passive immunotherapy has long been recognized as a promising treatment for bacterial infections like diphtheria, tetanus, and botulism. However, its use against resistant bacterial infections like Staphylococcus aureus is also anticipated (Keller and Stiehm 2000). This type of immunotherapy was first identified in 1893 and was introduced as an acceptable treatment about a century later, i.e., in 1996 (Schade and Hlinak 1996). This antibody has a protective activity lasting 5–10 years when stored at 4°C. It can last for 6 months when stored at room temperature and up to 1 month at a temperature of 37°C. For more extended storage, it is recommended to store it at −20°C or in powdered form (Larsson et al., 1993; Staak et al., 2001; Nilsson et al., 2012). One of the key benefits of this antibody is that it does not interact with host factors like the complement system, rheumatoid factor, and FC receptor. As a result, the immune system does not recognize it as a foreign object, and it does not cause any inflammatory complications (Schade et al., 2005). Another advantage of this immunotherapy method is its low probability of causing allergies in people. This is due to the fact that eggs are a natural part of the human diet. Therefore, allergic reactions are limited since egg allergies are primarily related to the albumin found in egg whites (Rahman et al., 2013). This immunotherapy method did not cause any complications during the ten years of treatment for Pseudomonas aeruginosa infection (Nilsson et al., 2007a,b). The evolutionary divergence between mammals and birds has also made them tend to bind to their target antigens even compared to mammalian IgG (Ikemori et al., 1993a,b; Ikemori et al., 1993a,b). IgY is found in high amounts in egg yolk. It is possible to isolate 60–150 mg of IgY per egg. Compared to serum antibodies from mammals such as mice or rabbits, IgY production is much more efficient. On average, each chicken can produce 22 g of IgY per year, of which only 10% is a specific antibody. In contrast, only 4 g of serum antibody can be isolated from rabbit blood. This method of producing IgY does not involve any stress or injury to the chickens, as the antibodies are separated from the eggs (Carlander et al., 1999a,b; Schade et al., 2005; Xu et al., 2011; Kovacs-Nolan and Mine, 2012; Li et al., 2015a,b). This treatment method has several advantages, such as being cost-effective, having a longer shelf life, and being non-invasive compared to other antibody-based treatments (Li et al., 2015a,b; Akbari et al., 2018). Compared to other antibodies with low sialic acid, IgY has high amounts of sialic acid and lacks the hook region in its structure, which increases its half-life and stability (Liu, 2015; Gilgunn, et al., 2016). Some studies have indicated that IgY does not cause side effects and can be used safely for long-term use, especially in sensitive populations such as children, pregnant women, and the elderly (Xu et al., 2018). Oral antibodies like IgY are more effective than intravenous antibodies like IgG, especially against bacterial toxins in gastrointestinal infections (Roberts et al., 2012). One of the benefits of this method is that it is highly effective. In laboratory conditions, it has been shown to completely inhibit the Helicobacter pylori bacteria when used at 16 mg/ml. However, the inhibitory effect is dependent on the concentration of antibodies. At low doses, this effect is not observed (Koo et al., 1999; Feng et al., 2013). IgY has some advantages over antibiotics. Unlike other antibiotics, IgY does not accumulate in poultry and livestock meat, meaning there are no restrictions on consuming such meat. Additionally, due to its natural properties, IgY does not cause environmental pollution (Li et al., 2015). This antibody can help immunocompromised patients, such as those undergoing chemotherapy treatment. These patients have an incomplete immune system and produce insufficient amounts of antibodies. This humoral immune deficiency can be resolved by introducing antibodies (Müller et al., 2015). IgY disadvantagesOne issue with this technology is that the production of IgY antibodies can be slow, taking up to 4 weeks to produce in chickens even under optimal conditions. Furthermore, certain studies have suggested that the presence of human antibodies against IgY may decrease the efficacy of this treatment (Díaz et al., 2014). The level of specific IgY in chickens increases significantly 28 days after antigen injection, reaching its peak on day 35 (Najdi et al., 2016). However, producing engineered monoclonal antibodies from polyclonal fragments may overcome these problems (Yamanaka et al., 1996). It is challenging to use oral IgY in patients due to its breakdown by the digestive system. Although many studies have been conducted to improve the IgY drug delivery system, it is still sensitive to gastric acidity and protease enzymes. Despite being more stable than other antibodies because of the absence of a hinge structure, IgY remains susceptible to degradation (Zhang et al., 2016). Several methods have been proposed to solve this problem, including using microbeads containing chicken antibodies and coating technologies such as alginate, calcium chitosan, and calcium pectinate (Sandolo et al., 2011; Wong et al., 2011; Yoshida et al., 2013; Bansal et al., 2014; Zhang et al., 2016). Carbonate buffer has been shown to protect IgY from degradation in the stomach of animal models (Roberts et al., 2012). Compounds like sorbitol, sucrose, and mannitol can stabilize this antibody against adverse temperature and acidity conditions (Müller et al., 2015). Research suggests that the unique hydrophobicity of IgY, when compared to IgG, could potentially enhance its stability. This intriguing characteristic of IgY, particularly when derived from egg yolk, may lead to improved patient outcomes compared to its purified form (Dávalos-Pantoja et al., 2000). However, a significant challenge with IgY is that its antibodies lack inhibitory properties once the Shiga toxin binds to the target. This limitation in efficacy, confined to initial use, underscores the need for further research and development (Neri et al., 2011). Caution is warranted when considering high doses of IgY, as studies on pigs have shown that excessive administration may lead to allergic reactions such as serum sickness. Furthermore, it can also trigger systemic and local responses, highlighting the importance of careful dosage management (Torché et al., 2006; Vega et al., 2012). Passive immunity, a viable treatment option, is not without its risks. The antibodies it generates, crucial for its effectiveness, have a limited lifespan in the patient’s blood. This necessitates a continuous supply of these antibodies to maintain their levels, a challenge that underscores the complexity of this treatment option. Large-scale production of these antibodies is, therefore, a necessity, a process that carries its own set of challenges (Chalghoumi et al., 2009). A study on the use of IgY in treating Acinetobacter baumannii pneumonia reported a surprising finding. Instead of leading to recovery, the treatment resulted in an increase in mortality and bacterial load in the mouse model. This unexpected outcome, known as antibody-dependent enhancement (ADE), raises significant concerns about the safety and efficacy of passive immunity. The researchers speculate that this may have been due to the shedding of the bacterial capsule (Jahangiri et al., 2021).
Fig. 1. Different hypotheses of IgY antibody response to bacterial infections designed by BioRender website (https://www.biorender.com/). Table 1. Advantages and disadvantages of immunoglobulin Y (IgY).
In a clinical trial, all participants reported mild side effects except for one who experienced severe nausea and vomiting (Jonsson et al., 2015). More investigations on the appropriate dosage and administration route can minimize patient complications. Table 1 lists the advantages and disadvantages of using IgY antibody. According to the above studies, the problems of using this antibody can be solved by eliminating side effects such as ADE through appropriate doses and routes of administration and recombinant antigens. In addition, determining the appropriate release methods and taking frequent doses increased its stability and effectiveness in the body. ConclusionThe excessive use of antibiotics and the emergence of antibiotic resistance have led to the development of new treatment technologies. One of these methods is the use of antibodies as passive immunity. Antibodies have a natural structure similar to human proteins, making them an effective tool in treating various microbial infections. Previous studies have shown that their side effects or minor disadvantages, such as ADE, can be eliminated with the help of bioinformatics methods and the production of recombinant antigens. However, more research is needed to determine their appropriate dose and route of administration for widespread use in various infections. AcknowledgmentsNot applicable. Conflict of interestThe authors state no conflicts of interest that could have influenced this work. FundingNone. Authors’ contributionsZ.E and S.K prepared the initial manuscript. K.G, M.K, and Z.E were responsible for drafting and editing the final article. K.G was the supervisor too. All authors have read and approved the final manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAkbari, M.R., Ahmadi, A., Mirkalantari, S. and J. Salimian 2018. “Anti-vibriocholerae IgY antibody inhibits mortality in suckling mice model.” J. Natl. Med. Assoc. 110(1), 84–87. Amro, W.A., Al-Qaisi, W. and Al-Razem, F. 2018. “Production and purification of IgY antibodies from chicken egg yolk.” J. Genet. Eng. Biotechnol. 16(1), 99–103. Bansal, V., Malviya, R., Malaviya. and Sharma, P.K. 2014. “Novel prospective in colon specific drug delivery system.” Polim. Med. 44(2), 109–118. Beaber, J.W., Hochhut, B. and Waldor, M.K. 2004. “SOS response promotes horizontal dissemination of antibiotic resistance genes.” Nature 427(6969), 72–74. Carlander, D., Stålberg, J. and Larsson, A. 1999a. “Chicken antibodies: a clinical chemistry perspective.” Ups. J. Med. Sci. 104(3), 179–189. Carlander, D., Sundstrom, J., Berglund, Å., Larsson, A., Wretlind, B. and Kollberg, H. 1999b. “Immunoglobulin Y (IgY) A new tool for the prophylaxis against Pseudomonas aeruginosa in cystic fibrosis patients.” Pediatr. Pulm. 18, 240. Chalghoumi, R., Marcq, C., Thewis, A., Portetelle, D. and Beckers, Y. 2009. “Effects of feed supplementation with specific hen egg yolk antibody (immunoglobin Y) on Salmonella species cecal colonization and growth performances of challenged broiler chickens.” Poult. Sci. 88(10), 2081–2092. Champion, J.A. and Mitragotri, S. 2006. “Role of target geometry in phagocytosis.” Proc. Natl. Acad. Sci. 103(13), 4930–4934. Dávalos Pantoja, L., Ortega Vinuesa, J.L., Bastos González, D. and Hidalgo Alvarez, R. 2000. “A comparative study between the adsorption of IgY and IgG on latex particles.” J. Biomater. Sci. Polym. Ed. 11(6), 657–673. Díaz, P., Malavé, C., Zerpa, N., Vázquez, H., ’Suze, G.D., Montero, Y., Castillo, C., Alagón, A. and Sevcik, C. 2014. “IgY pharmacokinetics in rabbits: implications for IgY use as antivenoms.” Toxicon 90, 124–133. Feng, Y., Liu, W. and Shi, D. 2013. “Effectiveness of egg yolk antibody against shiga toxin II variant toxicity in vitro and in vivo.” Curr. Microbiol. 67(4), 448–453. Gadde, U., Rathinam, T. and Lillehoj, H.S. 2015. “Passive immunization with hyperimmune egg-yolk IgY as prophylaxis and therapy for poultry diseases--a review.” Anim. Health. Res. Rev. 16(2), 163–176. Gilgunn, S., Millán Martín, S., Wormald, M.R., Zapatero-Rodríguez, J., Conroy, P.J., O’Kennedy, R.J., Rudd, P.M. and Saldova, R. 2016. “Comprehensive N-Glycan Profiling of Avian Immunoglobulin Y.” PLoS One 11(7), e0159859. Hamal, K.R., Burgess, S.C., Pevzner, I.Y. and Erf, G.F. 2006. “Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens.” Poult. Sci. 85(8), 1364–1372. Hofmann, T., Schmucker, S.S., Bessei, W., GrashornM. and Stefanski, V. 2020. “Impact of housing environment on the immune system in chickens: a review.” Animals 10; doi: 10.3390/ani10071138. Ikemori, Y., Peralta, R.C., Kuroki, M., Yokoyama, H. and Kodama, Y. 1993a. “Research note: avidity of chicken yolk antibodies to enterotoxigenic Escherichia coli fimbriae.” Poult. Sci. 72(12), 2361–2365. Ikemori, Y., Peralta, R.C., Kuroki, M., Yokoyama,H. and Kodama, Y. 1993b. “Research note: avidity of chicken yolk antibodies to enterotoxigenic Escherichia coli Fimbriae.” Poult. Sci. 72(12), 2361–2365. Jahangiri, A., Owlia, P., Rasooli, I., Salimian, J., Derakhshanifar, E., Aghajani, Z., Abdollahi, S., Khalili, S., Talei, D. and Eslam, E.D. 2021. “Specific egg yolk immunoglobulin as a promising non-antibiotic biotherapeutic product against Acinetobacter baumannii pneumonia infection.” Sci. Rep. 11(1), 1914. Joiner, K.A., Brown, E.J. and Frank, M.M. 1984. “Complement and bacteria: chemistry and biology in host defense.” Annu. Rev. Immunol. 2, 461–491. Jonsson, A.K., Larsson, A., Tängdén, T.,Melhus, Å. and Lannergård, A. 2015. “A trial with IgY chicken antibodies to eradicate faecal carriage of Klebsiella pneumoniae and Escherichia coli producing extended-spectrum beta-lactamases.” Infect. Ecol. Epidemiol. 5, 28224. Keller, M.A. and Stiehm, E.R. 2000. “Passive immunity in prevention and treatment of infectious diseases.” Clin. Microbiol. Rev. 13(4), 602–614. Klemperer, F. 1893. “Ueber natürliche Immunität und ihre Verwerthung für die Immunisirungstherapie.” Arch. Exp. Pathol. Pharmakol. 31(4), 356–382. Koo, J.K., Kim, C.H. and Choe, T.B. 1999. “Inhibition of growth and adhesion of Helicobacter pylori using egg yolk antibodies.” Biotechnol. Bioproc. Eng. 4(3), 219–223. Kovacs Nolan, J. and Mine, Y. 2012. “Egg yolk antibodies for passive immunity.” Annual Rev. Food. Sci. Technol. 3, 163–182. Larsson, A., Bålöw, R.M., Lindahl, T.L. and Forsberg, P.O. 1993. “Chicken antibodies: taking advantage of evolution--a review.” Poult. Sci. 72(10), 1807–1812. Lee, E.N., Sunwoo, H.H., Menninen, K. and Sim, J.S. 2002. “In vitro studies of chicken egg yolk antibody (IgY) against Salmonella enteritidis and Salmonella typhimurium.” Poult. Sci. 81(5), 632–641. Leiva, C.L., Cangelosi, A., Mariconda, V., Farace, M., Geoghegan, P., Brero, L., Fernández-Miyakawa, M. and Chacana, P. 2019. “IgY-based antivenom against Bothrops alternatus: production and neutralization efficacy.” Toxicon 163, 84–92. Li, X., Guo, Z., Li, J., Zhang, Y., Ma, H., Pang, X., Du, B. and Wei, Q. 2015a. “Quenched electrochemiluminescence of Ag nanoparticles functionalized g-C3N4 by ferrocene for highly sensitive immunosensing.” Anal. Chim. Acta. 854, 40–46. Li, X., Tang, C., Salama, M., Xia, M., Huang, X., Sheng, L. and Cai, Z. 2022. “Encapsulation efficiency and oral delivery stability of chitosan-liposome-encapsulated immunoglobulin Y.” J. Food. Sci. 87(4), 1708–1720. Li, X., Wang, L., Zhen, Y., Li, S. and Xu, Y. 2015b. “Chicken egg yolk antibodies (IgY) as non-antibiotic production enhancers for use in swine production: a review.” J. Anim. Sci. Biotechnol. 6(1), 40. Li, X., Yao, Y., Wang, X., Zhen, Y., Thacker, P.A., Wang, L., Shi, M., Zhao, J., Zong, Y., Wang, N. and Xu, Y. 2016. “Chicken egg yolk antibodies (IgY) modulate the intestinal mucosal immune response in a mouse model of Salmonella typhimurium infection.” Int. Immunopharmacol. 36, 305–314. Liu, L. 2015. “Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins.” J. Pharm. Sci. 104(6), 1866–1884. Mine, Y. and Kovacs-Nolan, J. 2002. “Chicken egg yolk antibodies as therapeutics in enteric infectious disease: a review.” J. Med. Food. 5(3), 159–169. Müller, S., Schubert, A., Zajac, J., Dyck, T. and Oelkrug, C. 2015. “IgY antibodies in human nutrition for disease prevention.” Nutr. J. 14, 109. Najdi, S., Nikbakht Brujeni, G., Sheikhi, N. and Chakhkar, S. 2016. “Development of anti-Helicobacter pylori immunoglobulins Y (IgYs) in quail.” Iran. J. Vet. Res. 17(2), 106–110. Neri, P., Tokoro, S., Kobayashi, R., Sugiyama, T., Umeda, K., Shimizu, T., Tsuji, T., Kodama, Y., Oguma, K. and Mori, H. 2011. “Specific egg yolk immunoglobulin as a new preventive approach for Shiga-toxin-mediated diseases.” PLoS One 6(10), e26526. Nilsson, E., Amini, A., Wretlind, B. and Larsson, A. 2007a. “Pseudomonas aeruginosa infections are prevented in cystic fibrosis patients by avian antibodies binding Pseudomonas aeruginosa flagellin.” J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 856(1-2), 75–80. Nilsson, E., Kollberg, H., Johannesson, M., Wejåker, P.E., Carlander, D. and Larsson, A. 2007b. “More than 10 years’ continuous oral treatment with specific immunoglobulin Y for the prevention of Pseudomonas aeruginosa infections: a case report.” J. Med. Food. 10(2), 375–378. Nilsson, E., Stålberg, J. and Larsson, A. 2012. “IgY stability in eggs stored at room temperature or at +4°C.” Br. Poult. Sci. 53(1), 42–46. Pereira, E.P.V., van Tilburg, M.F., Florean, E. and Guedes, M.I.F. 2019. “Egg yolk antibodies (IgY) and their applications in human and veterinary health: a review.” Int. Immunopharmacol. 73, 293–303. Pourhajibagher, M., Hashemi, F.B., Pourakbari, B., Aziemzadeh, M. and Bahador, A. 2016. “Antimicrobial resistance of Acinetobacter baumannii to imipenem in Iran: a systematic review and meta-analysis.” Open. Microbiol. J. 10, 32–42. Rahman, S., Van Nguyen, S., Icatlo Jr, F.C., Umeda, K. and Kodama, Y. 2013. “Oral passive IgY-based immunotherapeutics: a novel solution for prevention and treatment of alimentary tract diseases.” Hum. Vaccin. Immunother. 9(5), 1039–1048. Ren, H., Yang, W., Thirumalai, D., Zhang, X. and Schade, R. 2016. “A comparative evaluation of six principal IgY antibody extraction methods.” Altern. Lab. Anim. 44(1), 11–20. Roberts, A., McGlashan, J., Al-Abdulla, I., Ling, R., Denton, H., Green, S., Coxon, R., Landon, J. and Shone, C. 2012. “Development and evaluation of an ovine antibody-based platform for treatment of Clostridium difficile infection.” Infect. Immun. 80(2), 875–882. Rose, M.E., Orlans, E. and Buttress, N. 1974. “Immunoglobulin classes in the hen’s egg: their segregation in yolk and white.” Eur. J. Immunol. 4(7), 521–523. Sandolo, C., Péchiné, S., Le Monnier, A., Hoys, S., Janoir, C., Coviello, T., Alhaique, F., Collignon, A., Fattal, E. and Tsapis, N. 2011. “Encapsulation of Cwp84 into pectin beads for oral vaccination against Clostridium difficile.” Eur. J. Pharm. Biopharm. 79(3), 566–573. Savoldi, A., Carrara, E., Graham, D.Y., Conti, M. and Tacconelli, E. 2018. “Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions.” Gastroenterology 155(5), 1372–1382. Schade, R., Calzado, E.G., Sarmiento, R., Chacana, P.A., Porankiewicz-Asplund, J. and Terzolo, H.R. 2005. “Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine.” Altern. Lab. Anim. 33(2), 129–154. Schade, R. and Hlinak, A. 1996. “Egg yolk antibodies, State of the art and future prospects.” Altex 13(5), 5–9. Schwartz, F.A., Christophersen, L., Thomsen, K., Baekdal, S., Pals Bendixen, M., Jørgensen, M., Bull Rasmussen, I.K., Laulund, A.S., Høiby, N. and Moser, C. 2022. “Chicken IgY reduces the risk of Pseudomonas aeruginosa urinary tract infections in a murine model.” Front. Microbiol. 13, 988386. Shi, H., Zhu, J., Zou, B., Shi, L., Du, L., Long, Y., Wang, H., Xu, H., Zhen, Y. and Sun, L. 2017. “Effects of specific egg yolk immunoglobulin on pan-drug-resistant Acinetobacter baumannii.” Biomed. Pharmacother. 95, 1734–1742. Shikun Ge, Y.Y., Brindha, C., Antonysamy M., Fagang Z. and Xiaoying Z. 2020. “Evaluation of different IgY preparation methods and storage stability as potential animal feed supplement.” Pakistan. J. Zool. 52, 2305–2311 Siriya, P., Chu, C., Chen, M.T., Lo, C.C., Huang, S.L. and Lien, T.F. 2013. “Extraction and purification of anti-Helicobacter pylori IgY.” J. Agri. Sci. 5(3), 132. Staak, C., Schwarzkopf, C., Behn, I., Hommel, U., Hlinak, A., Schade, R. and Erhard, M. 2001. Isolation of IgY from yolk. Chicken egg yolk antibodies, production and application: IgY-technology. 65–107. Sun, S., Mo, w., Ji, Y. and Liu, S. 2001. “Preparation and mass spectrometric study of egg yolk antibody (IgY) against rabies virus.” Rapid. Commun. Mass. Spectrom. 15(9), 708–712. Sunwoo, H.H., Lee, E.N., Menninen, K., Suresh, M.R. and Sim, J.S. 2002. “Growth inhibitory effect of chicken egg yolk antibody (IgY) on Escherichia coli O157:H7.” J. Food. Sci. 67(4), 1486–1494. Taylor, A.I., Fabiane, S.M., Sutton, B.J. and Calvert, R.A. 2009. “The crystal structure of an avian IgY-Fc fragment reveals conservation with both mammalian IgG and IgE.” Biochemistry 48(3), 558–562. Thomsen, K., Christophersen, L., Bjarnsholt, T., Jensen, P., Moser, C. and Høiby, N. 2015. “Anti-Pseudomonas aeruginosa IgY antibodies induce specific bacterial aggregation and internalization in human polymorphonuclear neutrophils.” Infect. Immun. 83(7), 2686–2693. Thu, H.M., Myat, T.W., Win, M.M., Thant, K.Z., Rahman, S., Umeda, K., Nguyen, S.V., Icatlo, F.C., Higo-Moriguchi, Jr., K., Taniguchi, K.., Tsuji, T., Oguma, K., Kim, S.J., Bae, H.S. and Choi, H.J. 2017. “Chicken egg yolk antibodies (IgY) for prophylaxis and treatment of rotavirus diarrhea in human and animal neonates: a concise review.” Korean. J. Food. Sci. Anim. Resour. 37(1), 1–9. Torché, A.M., Le Dimna, M., Le Corre, P., Mesplède, A., Le Gal, S., Cariolet, R. and Le Potier, M.F. 2006. “Immune responses after local administration of IgY loaded-PLGA microspheres in gut-associated lymphoid tissue in pigs.” Vet. Immunol. Immunopathol. 109(3-4), 209–217. Tsubokura, K., Berndtson, E., Bogstedt, A., Kaijser, B., Kim, M., Ozeki, M. and Hammarström,L. 1997. “Oral administration of antibodies as prophylaxis and therapy in Campylobacter jejuni-infected chickens.” Clin. Exp. Immunol. 108(3), 451–455. Vega, C.G., Bok, M., Vlasova, A.N., Chattha, K.S., Fernández, F.M., Wigdorovitz, A., Parreño, V.G. and Saif, L.J. 2012. “IgY antibodies protect against human Rotavirus induced diarrhea in the neonatal gnotobiotic piglet disease model.” PLoS One 7(8), e42788. Warr, G.W., Magor, K.E. and Higgins, D.A. 1995. “IgY: clues to the origins of modern antibodies.” Immunol. Today. 16(8), 392–398. Wen, J., Zhao, S., He, D., Yang, Y., Li,Y. and Zhu, S. 2012. “Preparation and characterization of egg yolk immunoglobulin Y specific to influenza B virus.” Antiviral. Res. 93(1), 154–159. Wong, T.W., Colombo, G. and Sonvico, F. 2011. “Pectin matrix as oral drug delivery vehicle for colon cancer treatment.” AAPS PharmSciTech 12(1), 201–214. Xu, Y., Li, X., Jin, L., Zhen, Y., Lu, Y., Li, S., You, J. and Wang, L. 2011. “Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: a review.” Biotechnol. Adv. 29(6), 860–868. Xu, Y., Selerio-Poely, T. and Ye, X. 2018. “Clinical and microbiological effects of egg yolk antibody against Porphyromonas gingivalis as an adjunct in the treatment of moderate to severe chronic periodontitis: a randomized placebo-controlled clinical trial.” J. Periodontal. Implant. Sci. 48(1), 47–59. Yamanaka, H.I., Inoue, T. and Ikeda-Tanaka, O. 1996. “Chicken monoclonal antibody isolated by a phage display system.” J. Immunol. 157(3), 1156–1162. Yokoyama, H., Peralta, R.C., Sendo, S., Ikemori, Y. and Kodama, Y. 1993. “Detection of passage and absorption of chicken egg yolk immunoglobulins in the gastrointestinal tract of pigs by use of enzyme-linked immunosorbent assay and fluorescent antibody testing.” Am. J. Vet. Res. 54(6), 867–872. Yoshida, T., Lai, T.C., Kwon, G.S. and Sako, K. 2013. “pH- and ion-sensitive polymers for drug delivery.” Expert. Opin. Drug. Deliv. 10(11), 1497–1513. Zhang, S., Xing, P., Guo, G., Liu, H., Lin, D., Dong, C., Li, M. and Feng, D. 2016. “Development of microbeads of chicken yolk antibodies against Clostridium difficile toxin A for colonic-specific delivery.” Drug. Deliv. 23(6), 1940–1947. | ||
| How to Cite this Article |
| Pubmed Style Esmaeili Z, Shahsavar SK, Keikha M, Ghazvini K. Chicken immunoglobulin (IgY) as an alternative treatment for bacterial infections, emphasizing advantages, disadvantages and mechanisms. J Microbiol Infect Dis. 2024; 14(3): 95-102. doi:10.5455/JMID.2024.v14.i3.2 Web Style Esmaeili Z, Shahsavar SK, Keikha M, Ghazvini K. Chicken immunoglobulin (IgY) as an alternative treatment for bacterial infections, emphasizing advantages, disadvantages and mechanisms. https://www.jmidonline.org/?mno=206671 [Access: January 25, 2026]. doi:10.5455/JMID.2024.v14.i3.2 AMA (American Medical Association) Style Esmaeili Z, Shahsavar SK, Keikha M, Ghazvini K. Chicken immunoglobulin (IgY) as an alternative treatment for bacterial infections, emphasizing advantages, disadvantages and mechanisms. J Microbiol Infect Dis. 2024; 14(3): 95-102. doi:10.5455/JMID.2024.v14.i3.2 Vancouver/ICMJE Style Esmaeili Z, Shahsavar SK, Keikha M, Ghazvini K. Chicken immunoglobulin (IgY) as an alternative treatment for bacterial infections, emphasizing advantages, disadvantages and mechanisms. J Microbiol Infect Dis. (2024), [cited January 25, 2026]; 14(3): 95-102. doi:10.5455/JMID.2024.v14.i3.2 Harvard Style Esmaeili, Z., Shahsavar, . S. K., Keikha, . M. & Ghazvini, . K. (2024) Chicken immunoglobulin (IgY) as an alternative treatment for bacterial infections, emphasizing advantages, disadvantages and mechanisms. J Microbiol Infect Dis, 14 (3), 95-102. doi:10.5455/JMID.2024.v14.i3.2 Turabian Style Esmaeili, Zahra, Sara Kamal Shahsavar, Masoud Keikha, and Kiarash Ghazvini. 2024. Chicken immunoglobulin (IgY) as an alternative treatment for bacterial infections, emphasizing advantages, disadvantages and mechanisms. Journal of Microbiology and Infectious Diseases, 14 (3), 95-102. doi:10.5455/JMID.2024.v14.i3.2 Chicago Style Esmaeili, Zahra, Sara Kamal Shahsavar, Masoud Keikha, and Kiarash Ghazvini. "Chicken immunoglobulin (IgY) as an alternative treatment for bacterial infections, emphasizing advantages, disadvantages and mechanisms." Journal of Microbiology and Infectious Diseases 14 (2024), 95-102. doi:10.5455/JMID.2024.v14.i3.2 MLA (The Modern Language Association) Style Esmaeili, Zahra, Sara Kamal Shahsavar, Masoud Keikha, and Kiarash Ghazvini. "Chicken immunoglobulin (IgY) as an alternative treatment for bacterial infections, emphasizing advantages, disadvantages and mechanisms." Journal of Microbiology and Infectious Diseases 14.3 (2024), 95-102. Print. doi:10.5455/JMID.2024.v14.i3.2 APA (American Psychological Association) Style Esmaeili, Z., Shahsavar, . S. K., Keikha, . M. & Ghazvini, . K. (2024) Chicken immunoglobulin (IgY) as an alternative treatment for bacterial infections, emphasizing advantages, disadvantages and mechanisms. Journal of Microbiology and Infectious Diseases, 14 (3), 95-102. doi:10.5455/JMID.2024.v14.i3.2 |