| Research Article | ||
J. Microbiol. Infect. Dis., (2024), Vol. 14(1): 21–29 Original Research Diagnostic significance of bronchoalveolar lavage galactomannan assay in invasive aspergillosis: A comprehensive analysisNairita Choudhuri1, Bhaskar Narayan Chaudhuri2*, Partha Guchhait2, Anupam Das2 and Satadal Das21Vellore Institute of Technology, Vellore, India 2Peerless Hospitex Hospital and Research Center Limited, Kolkata, India *Corresponding Author: Bhaskar Narayan Chaudhuri. Peerless Hospitex Hospital and Research Center Limited, Kolkata, India. Email: bhaskarnchau [at] gmail.com Submitted: 07/12/2023 Accepted: 24/03/2024, Published: 31/03/2024 © 2024 Journal of Microbiology and Infectious Diseases
AbstractBackground: In critically ill and immunocompromised patients, diagnosing invasive aspergillosis (IA) is crucial. This study focuses on the Galactomannan (GM) antigen test. The study categorizes GM-positive patients into two groups based on bronchoalveolar lavage (BAL) fluid GM levels: group 1 (GM index >1.5) and group 2 (GM index >0.5–1.5). Aim: This study aims to investigate the association between GM levels in BAL fluid and clinical outcomes in IA patients, specifically focusing on mortality rates. In addition, the study explores the role of C-reactive protein (CRP) levels, type 2 diabetes mellitus, and chronic kidney disease (CKD) as key risk factors for IA. Methods: Patients with GM-positive results were divided into group 1 and group 2 based on GM index levels. The study assessed age, sex distribution, and mortality rates in these groups. Results: While there were no significant differences in age and sex distribution between group 1 and 2, a higher mortality rate was observed in group 1, aligning with established clinical expectations. The study highlights the importance of categorizing GM interpretations based on these two groups. CRP levels, along with comorbidities such as type 2 diabetes mellitus and CKD, emerged as key risk factors for IA. Conclusion: This research emphasizes the significance of GM levels in BAL fluid as a prognostic indicator for IA, especially when categorized into GM index groups. The study contributes valuable insights into mortality rates and identifies CRP levels, type 2 diabetes mellitus, and CKD as crucial risk factors. These findings enhance our understanding of IA diagnostics and risk stratification. Keywords: Aspergillus, Diagnostic Tests, Enzyme-Linked Immunosorbent Assay, Mycoses. IntroductionIn the realm of Medical Mycology, fungi play diverse roles, from being ubiquitous in the environment to forming part of the normal flora in humans and animals. While many fungal interactions are symbiotic or commensal, some instances lead to a spectrum of infections, ranging from mild superficial conditions to severe and life-threatening invasive infections. Invasive fungal infections are specifically defined as those where fungi invade deep tissues, leading to prolonged illness (Ramana et al., 2013). IFIs, particularly invasive Aspergillosis (IA), have become a significant concern, especially among immunocompromised and critically ill patients. The prevalence of IFIs, encompassing various fungal species, has seen a surge due to increased immunosuppression in recent years (Xess et al., 2022). This paper primarily focuses on the diagnosis of IA, a condition predominantly affecting severely immunocompromised individuals, including those with acute myeloid leukemia, prolonged neutropenia, or recipients of hematopoietic cell or solid organ transplants (Mercier et al., 2018). It is estimated to affect around 250,900 patients per year in India (Ray et al., 2022). Several diagnostic methods are available for IA, including polymerase chain reaction (PCR), Beta-D-Glucan Test, and the Galactomannan (GM) Antigen Test. PCR identifies specific Aspergillus DNA sequences and is applicable to diverse sample types including blood, respiratory specimens, and tissue biopsies (Kami et al., 2001). The Beta-D-Glucan Test detects the presence of beta-D-glucan, a fungal cell wall component, in the blood (De Vlieger et al., 2011). However, the GM Antigen Test takes center stage in this paper. Operating on the principle of identifying the presence of GM, a polysaccharide in the cell wall of Aspergillus spp., this test has emerged as a pivotal biomarker for IA diagnosis(Mercier et al., 2018). The test also has different cutoff values for various specimens, such as serum (0.5–0.7 ng/ml), urine, bronchoalveolar lavage (BAL) fluid (1 ng/ml), and pericardial fluid and cerebrospinal fluid (CSF) (0.5 ng/ml) (Quindós et al., 2014). In terms of physical and chemical characteristics, GM is a key immunodominant hetero-polysaccharide component found in the cell walls of Aspergillus and Penicillium species. It was the first antigen identified in both animal models and patients with IA (Thornton, 2010). It features a mannose backbone with varying numbers of galactofuran side chains, exhibiting sizes ranging from 35 to 200 kDa (Mercier et al., 2018). GM can be released into the blood and other body fluids, even in the early stages of Aspergillus invasion. GM is a significant constituent released by Aspergillus species, particularly during active cell replication, such as during hyphal growth (Karapinar, 2018). GM detection in various matrices, including CSF (Chong et al., 2016), urine and plasma (Duettmann et al., 2014), and broncho-alveolar lavage (BAL) fluid, has been reported. This antigen is detected through enzyme-linked immunosorbent assay (ELISA) or lateral flow assays and clinical samples such as serum (blood) and BAL fluid are most utilized for testing. Several studies have notably observed that BAL GM testing when compared to serum GM testing is a reasonably safer diagnostic method, exhibiting higher sensitivity and specificity, particularly in patients at risk with hematological diseases, Chronic Pulmonary Aspergillosis, and invasive pulmonary Aspergillosis (IPA) (Gupta et al., 2017; Sehgal et al., 2019; Wu et al., 2021)testing for values of galactomannan (GM). Hence, in our study, solely BAL fluid samples have been tested. Elevated levels of GM may signify an active Aspergillus infection, aiding clinicians in timely and accurate diagnosis. In the paper by Latgé et al. (1994), the immunoreactivity of GM and HCl-hydrolyzed GM was investigated using sera from Aspergillosis patients and an antigalactofuran monoclonal antibody through various assays, including ELISA and immunodiffusion assays. A variability in response was observed in different immunological assays which emphasizes the importance of carefully selecting and validating assay methods for accurate detection of GM in clinical settings. The test’s utility is further enhanced when employed in conjunction with other diagnostic modalities and clinical indicators of disease. The ability to detect elevated levels of GM in clinical samples signifies active Aspergillus infection, providing clinicians with a valuable tool for timely and accurate diagnosis. This paper seeks to contribute novel insights to the field of IA diagnosis. By exploring the immunoreactivity of GM and scrutinizing the variability in assay methods, we aim to advance our understanding of IA diagnostics providing a comprehensive overview of the clinical importance and intricacies of GM testing in the diagnosis of IA. Materials and MethodsMaterialsBronchoscopies with BAL fluid, conducted by professional recommendations, were carried out for 19 patients at the Peerless Hospitex Hospital and Research Center Limited, Kolkata, India. All patients were clinically evaluated for IA as per EORTC-MSG (European Organization for Research and Treatment of Cancer/Mycosis Study group) guidelines into proven, probable or possible IA. To ensure optimal preservation, all BAL samples were stored at −70°C. As per our departmental (National accredited lab) standard operative procedure, we have to run PCR on the same day of sample collection for Aspergillus; however, the GM ELISA test is used to run for 3 days in a week. The study was done as per Institutional Ethical Committee guidelines with strict instructions for only data analysis without revealing the identity of the subject (no. PHH and RCL/CREC/FM02). Other than the GM test we used to perform routine culture for Aspergillus on Sabouraud’s dextrose agar medium followed by the study of the colonial characters, lactophenol cotton blue morphological study, and PCR study in which we could confirm three Aspergillus species- A. fumigatus, A. flavus and A. terreus; remaining Aspergillus species are reported as Aspergillus spp. other than these three species. In these 19 cases, we could identify four A. fumigatus and two cases of A flavus and other Aspergillus spp. were identified only at Genus level. Due to this species correlation with GM levels could not be done. BAL fluid Aspergillus GM levels were assessed using the platelia enzyme immunoassay (EIA) kit from Bio-Rad Laboratories, Munich, Germany, as part of a clinical routine at the Peerless Hospitex Hospital and Research Centre Limited, Kolkata, India. The determination was conducted following the manufacturer’s instructions and processed in adherence to the provided protocol. While our primary analysis centered around the recommended 1.0 optical density index (ODI) cut-off for BALF, an additional evaluation was performed using a 0.80 GM ODI cut-off. GM TestThe Bio-Rad Platelia Aspergillus GM antigen sandwich EIA (GM-EIA) is commonly used for screening and prospective surveillance of IA in high-risk patients (Gorton et al., 2015). The assay is based on a one-stage immunoenzymatic sandwich microplate assay. It utilizes rat EBA-2 monoclonal antibodies, which specifically target Aspergillus GM by selectively binding to four or more β(1 → 5) galactofuranosyl residues of GM (Mennink-Kersten et al., 2004). This concept is backed by the paper by Stynen et al., 1992 which describes the design of monoclonal antibodies that are highly specific for Aspergillus fumigatus GM and confirms that their characteristics make them promising tools for the diagnosis of IA through antigen detection. Table 1. Details of the patients with galactomannan levels more than 1.5 index value.
Monoclonal antibodies (EBA-2) are used for two purposes: For coating the wells of the microplate to immobilize the Aspergillus GM antigen, and for the detection of the antigen bound to the sensitized microplate, which is done by another set of peroxidase-linked monoclonal antibodies. For sample preparation, the serum or BAL fluid samples are heat-treated in the presence of EDTA. This causes dissociation of immune complexes and precipitation of proteins that could potentially interfere with the test. The treated samples and the conjugate (peroxidase-linked monoclonal antibodies) are added to the microplate wells coated with monoclonal antibodies. The plate is then incubated, allowing the formation of a complex between the monoclonal antibodies, GM antigen, and peroxidase and after that, the microplate strips are washed to remove any unbound material. This is followed by the addition of chromogen TMB solution to the wells. This solution reacts with the complexes bound to the well, resulting in a blue color reaction. The enzyme reaction is stopped by the addition of acid, leading to a change in the blue color to yellow. Then, the absorbance optical density (OD) of specimens and controls is determined using a spectrophotometer, and measurements are taken at wavelengths of 450 nm and 620/630 nm. The resulting absorbance values provide information about the concentration of Aspergillus GM in the samples, which is then used in the diagnosis of Aspergillus infections. In this study, we selected nine patients with GM index value of more than 1.5 and ten patients with GM index value >0.5–1.5 and then compared their clinical profile, treatment outcome, associated co-morbidities, and C-reactive protein (CRP) levels in the blood. Table 2. Details of the patients with Galactomannan level 0.5–1.5 index value.
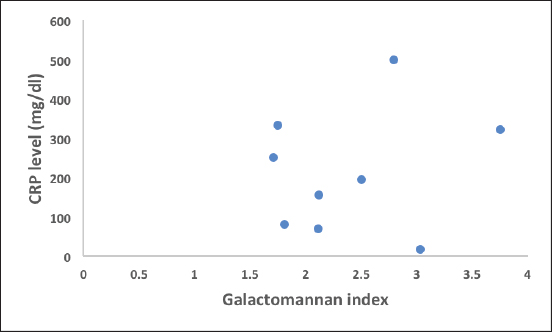
Ethical approvalEthical approval was taken from the Institutional Ethical Committee for this retrospective study. ResultsGroup 1. Details of the patients with GM levels of more than 1.5 index valueDetails of the patients with GM levels of more than 1.5 index value are given in Table 1. Group 2. Details of the patients with GM level 0.5–1.5 index valueDetails of the patients with GM level 0.5–1.5 index value are given in Table 2. It was important to note that in group 1, three patients were proven IA and among them two patients died, remaining six patients belonged to the probable IA group, and among them, only one patient died. In group 2, two patients were proven IA and both of them survived. Six cases were probable IA, and among them, one patient died. The remaining two patients belonged to possible IA and both of them survived. The difference in GM index value between two groups is statistically highly significant validating our study design with a p value <0.0001. The age distribution is uniform in the two groups with a range of 47–80 years of age; the difference between the two groups is not statistically significant with a p value of 0.7597. Males are predominant (~80%). Type 2 diabetes mellitus, chronic kidney disease, and high CRP levels (>150 mg/dl) are the main determining factors of mortality in both groups. Similarly clinically proven IA patients also showed high mortality. A high mortality rate (33.33%) is associated with a GM index value of more than 1.5. Although a positive correlation exists between GM index value and the CRP level in blood the relation is very weak (Figs. 1–3). Details of the findings are given in Tables 3 and 4.
Fig. 1. Scatterogram of galactomannan index (>1.5) and CRP level (correlation value R=0.1949).
Fig. 2. Scatterogram of galactomannan index (>0.5–1.5) and CRP level (correlation value R=0.3278). DiscussionFalse positive and risk factorsOur results, obtained through the GM-EIA, contribute to the growing body of knowledge on IA diagnosis. We aim to address the reliability and significance of the GM assay. Regular use of the test may contribute to the earlier diagnosis of IA in some patients. GM assay serves as a predictive indicator for a higher risk of death in individuals with IA in the context of acute leukemia and hematopoietic stem cell transplantation among other diseases (Ghosh et al., 2013). Positive findings suggest invasive disease, but false positives can occur, especially during the neutropenic period post-HSCT (Steinbach, 2012). Moreover, certain studies confirm that BAL fluid-GM may be more useful as compared to serum in diagnosing in selected patients (Zhou et al., 2017 Sehgal et al., 2019).
Fig. 3. Scatterogram of galactomannan index (>0.5) and CRP level (correlation value R=0.3018) Despite its widespread use, the diagnostic performance of the GM-EIA often yields false-positive results which have been associated with factors such as antimicrobial treatment, other invasive fungal diseases, and even specific ingestions. In pediatric cases, earlier studies suggested higher false positives(Herbrecht et al., 2002). However, recent studies show the GM assay is effective in children with a low false-positive rate (Fisher et al., 2012). Caution is also needed with certain antibiotics causing false positives. For example, false-positive results may be associated with concurrent piperacillin-tazobactam treatment (Pfeiffer et al., 2006), but recent literature indicates that this interaction may also no longer be a concern. A study in Hospira Healthcare India Private Limited indicated no drug–laboratory interaction with piperacillin/tazobactam and a potential interaction with imipenem/cilastatin (Otting et al., 2014). Sensitivity of GM assay is also recorded to be lower in solid-organ transplant recipients than in those with hematologic malignancies or HSCT(Husain and Camargo, 2019; Pfeiffer et al., 2006; Sigera and Denning, 2023). The administration of intravenous immunoglobulin can also potentially result in significant false-positive results in the GM-EIA, even in patients at a high risk of IA (Liu et al., 2020). From our study, we understand that GM assay testing, especially twice a week, is recommended, and an increase during the first week may predict progressive disease as there is no doubt that GM detection precedes clinical symptoms by days. Given the high mortality rates associated with IPA in the patient population, it is also important to define clinically relevant cut-off values for GM for effective clinical practice (Bukkems et al., 2023). The positive predictive value (PPV) of the GM BAL fluid assay is reported to be excellent (100%) when using an OD index cutoff of ≥3 but when a lower cutoff of ≥0.5 is employed, the PPV drops to a moderate level of 76.4% (D’Haese et al., 2012). In a more recent paper, the identified optimal cutoff value of 0.88 demonstrated good diagnostic performance, balancing sensitivity and specificity (Lai et al., 2020). The overarching goal is to refine diagnostic strategies for IA, ultimately improving patient outcomes. Assessment of the response to treatment in IA is challenging. GM index value >1.4 if it fails to convert to 0.5 after 1 week is characterized by a high mortality rate (48.1%), and this mortality rate becomes 10.1% if it converts to 0.5% (Mercier et al., 2020). Peculiarly these proportions correspond to the mortality rates of our group 1 and group 2, respectively. This also validates interpretations based on the GM test in two categories defined by us. Another interesting finding in our study is that CKD is an important risk factor in IA, as in most of the previous reports this was not indicated. In this report, we find IA affecting mainly the elderly age group (47–80 years.) although in most preceding reports lower age groups were reported suffering from IA including the pediatric age group. Significant male prevalence is again curiously alike with nearly all previous studies. The reason for this is unidentified. Finally, it should be noted that while our study provides valuable insights into the diagnostic potential of GM assay in IA, the limited number of positive results (19 cases) underscores the need for cautious interpretation and a potential gap for future research. Table 3. Matrix of the findings of group 1.
Table 4. Matrix of the findings of group 2.
Standardization and clinical importance of GM testThe GM detection assay, particularly the platelia Aspergillus assay, is well standardized, which ensures consistency and reliability of results across different laboratories and settings. The assay requires only small amounts of specimen for analysis, typically 300 μl of the patient’s blood, and has a relatively fast turnaround time, taking approximately 4 hours to provide results (Quindós et al., 2014). This is advantageous as it not only minimizes the volume of blood needed for testing but also allows for early diagnosis and timely initiation of treatment. There is still, however, considerable variability in the performance of the GM assay across different studies, especially in terms of sensitivity, which may range from 30%–100% in the case of serum while specificity may be above 75% (Karapinar, 2018). For example, the paper by Moragues et al., 2003, observed a sensitivity of 66.7% for proven IA and 50% for probable IA, while the sensitivity was observed at 98% or higher in all risk groups studied. The meta-analysis results by Pfeiffer et al., 2006 indicated that the GM test’s efficacy in cases of proven IA demonstrated a sensitivity of 71% and a specificity of 89%. The observed differences in test results may be contributed to factors such as the choice of cut-off points and variations in study populations. The article by Sun et al., 2010 confirms that the sensitivity of the GM test decreases with a rise in the cut-off value, while specificity increases. They also mention that the rate of underdiagnosis of serum GM detection was reported as 34%, and the rate of misdiagnosis was 10%. The overall sensitivity and specificity of the GM detection assay are reported to be in the range of 65%–75% and 80%–95%, respectively (Quindós et al., 2014), which positions it as a supportive tool in the diagnostic process. The test’s sensitivity and specificity make it a valuable tool, aiding clinicians in identifying IA even before the onset of clinical symptoms or visible radiological evidence. Moreover, the GM test assists in monitoring treatment response, with declining levels correlating with a positive therapeutic outcome. Despite some variability in performance influenced by factors like antifungal therapy, its standardized nature, speed, and reliability make the GM test an indispensable component of the diagnostic arsenal for managing IA, particularly in high-risk patient populations. ConclusionOur study explores the importance of frequent GM assay testing, especially in high-risk populations, to predict and monitor IA progression. Clinically relevant cutoff values, such as the recently identified 0.88, offer a balanced diagnostic approach. Notably, the observed mortality rates align with distinct GM index categories, validating their clinical significance. In addition, our findings highlight chronic kidney disease as a previously underappreciated risk factor for IA, emphasizing the need for comprehensive risk assessment. The age distribution and male prevalence in IA further contribute to the nuanced understanding of this complex condition. Overall, refining diagnostic strategies and recognizing key risk factors are crucial steps toward improving patient outcomes in the challenging landscape of IA. AcknowledgementThe authors thank the Peerless Hospitex Hospital and Research Center Limited, West Bengal, India, for providing all patient samples. Conflict of interestThe authors declare that they have no conflict of interest. FundingNo funding. Self-financed. Authors contributionNairita Choudhuri contributed to data collection and analysis, and the manuscript writing. Bhaskar Naryan Chaudhuri was involved in experiment design, data analysis, and manuscript correction. Partha Guchhait assisted in manuscript correction. Anupam Das was responsible for collecting and analyzing all hospital records. Satadal Das contributed to experimental design and patient data analysis. Data availabilityAll data are provided in the manuscript. ReferencesBukkems, L.M.P., Van Dommelen, L., Regis, M., Van Den Heuvel, E. and Nieuwenhuizen, L. 2023. The use of galactomannan antigen assays for the diagnosis of invasive pulmonary aspergillosis in the hematological patient: a systematic review and meta-analysis. J. Fungi. 9(6), 674. Chong, G.M., Maertens, J.A., Lagrou, K., Driessen, G.J., Cornelissen, J.J. and Rijnders, B.J. A. 2016. Diagnostic performance of galactomannan antigen testing in cerebrospinal fluid. J. Clin. Microbiol. 54(2), 428–431. De Vlieger, G., Lagrou, K., Maertens, J., Verbeken, E., Meersseman, W. and Van Wijngaerden, E. 2011. Beta- d -glucan detection as a diagnostic test for invasive aspergillosis in immunocompromised critically Ill patients with symptoms of respiratory infection: an autopsy-based study. J. Clin. Microbiol. 49(11), 3783–3787. D’Haese, J., Theunissen, K., Vermeulen, E., Schoemans, H., De Vlieger, G., Lammertijn, L., Meersseman, P., Meersseman, W., Lagrou, K. and Maertens, J. 2012. Detection of galactomannan in bronchoalveolar lavage fluid samples of patients at risk for invasive pulmonary aspergillosis: analytical and clinical validity. J. Clin. Microbiol. 50(4), 1258–1263. Duettmann, W., Koidl, C., Troppan, K., Seeber, K., Buzina, W., Wolfler, A., Wagner, J., Krause, R. and Hoenigl, M. 2014. Serum and urine galactomannan testing for screening in patients with hematological malignancies. Med. Mycol. 52(6), 647–652. Fisher, B.T., Zaoutis, T.E., Park, J.R., Bleakley, M., Englund, J.A., Kane, C., Arceci, R.J., Guinan, E., Smith, F.O., Luan, X. and Marr, K.A. 2012. Galactomannan antigen testing for diagnosis of invasive aspergillosis in pediatric hematology patients. J. Pediat. Infect. Dis. Soc. 1(2), 103–111. Ghosh, I., Raina, V., Kumar, L., Sharma, A., Bakhshi, S. and Iqbal, S. 2013. Serum galactomannan assay for diagnosis of probable invasive aspergillosis in acute leukemia and hematopoietic stem cell transplantation. Indian J. Med. Paediat. Oncol. 34(2), 74–79. Gorton, R.L., White, P.L., Bagkeris, E., Cotterall, D., Desai, R., McHugh, T. and Kibbler, C.C. 2015. Improved standardization of the bio-rad platelia Aspergillus galactomannan antigen sandwich enzyme immunoassay using the DS2 (Dynex) enzyme-linked immunosorbent assay (ELISA) processing system. J. Clin. Microbiol. 53(7), 2072–2078. Gupta, A., Capoor, M.R., Shende, T., Sharma, B., Mohindra, R., Suri, J.C. and Gupta, D.K. 2017. Comparative evaluation of galactomannan test with bronchoalveolar lavage and serum for the diagnosis of invasive aspergillosis in patients with hematological malignancies. J. Lab. Physicians. 9(4), 234–238. Herbrecht, R., Letscher-Bru, V., Oprea, C., Lioure, B., Waller, J., Campos, F., Villard, O., Liu, K.-L., Natarajan-Amé, S., Lutz, P., Dufour, P., Bergerat, J.-P. and Candolfi, E. 2002. Aspergillus galactomannan detection in the diagnosis of invasive aspergillosis in cancer patients. J. Clin. Oncol. 20(7), 1898–1906. Husain, S. and Camargo, J.F. 2019. Invasive aspergillosis in solid-organ transplant recipients: guidelines from the American Society of transplantation infectious diseases Community of practice. Clin. Transplant. 33(9), e13544. Kami, M., Fukui, T., Ogawa, S., Kazuyama, Y., Machida, U., Tanaka, Y., Kanda, Y., Kashima, T., Yamazaki, Y., Hamaki, T., Mori, S., Akiyama, H., Mutou, Y., Sakamaki, H., Osumi, K., Kimura, S. and Hirai, H. 2001. Use of real-time PCR on blood samples for diagnosis of invasive aspergillosis. Clin. Infect. Dis. 33(9), 1504–1512. Karapinar, D. 2018. A review of a diagnostic tool: galactomannan. J. Immunol. Sci. 2(5), 38–42. Lai, G., Zeng, C., Mo, J., Song, W. and Xu, P. 2020. Diagnostic value of galactomannan in bronchoalveolar lavage fluid for chronic respiratory disease with pulmonary aspergillosis. J. Clin. Microbiol. 58(3), e01308–19. Latgé, J. P., Kobayashi, H., Debeaupuis, J. P., Diaquin, M., Sarfati, J., Wieruszeski, J. M., Parra, E., Bouchara, J. P. and Fournet, B. 1994. Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus. Infect. Immun. 62(12), 5424–5433. Liu, W.D., Lin, S.W., Shih, M.C., Su, C.L., Wang, Y.W., Lin, S.C., Lee, Y.F., Huang, H.H., Chou, W.C., Wu, U.I., Chen, Y.C. and Chang, S.C. 2020. False-positive Aspergillus galactomannan immunoassays associated with intravenous human immunoglobulin administration. Clin. Microbiol. Infect. 26(11), 1555.e9–1555.e14. Mennink-Kersten, M.A., Donnelly, J.P. and Verweij, P.E. 2004. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect. Dis. 4(6), 349–357. Mercier, T., Guldentops, E., Lagrou, K. and Maertens, J. 2018. Galactomannan, a surrogate marker for outcome in invasive aspergillosis: finally coming of age. Front. Microbiol. 9, 661. Mercier, T., Wera, J., Chai, L.Y.A., Lagrou, K. and Maertens, J. 2020. A mortality prediction rule for hematology patients with invasive aspergillosis based on serum galactomannan kinetics. J. Clin. Med. 9(2), 610. Moragues, M.D., Amutio, E., García-Ruiz, J.C. and Pontón, J. 2003. Usefulness of galactomannan detection in the diagnosis and follow-up of hematological patients with invasive aspergillosis. Rev Iberoam Micol. 20(3), 103–110. Otting, K.A., Stover, K.R. and Cleary, J.D. 2014. Drug–laboratory interaction between beta-lactam antibiotics and the galactomannan antigen test used to detect mould infections. Brazilian J. Infect. Dis. 18(5), 544–547. Pfeiffer, C.D., Fine, J.P. and Safdar, N. 2006. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin. Infect. Dis. 42(10), 1417–1727. Quindós, G., Eraso, E., Ezpeleta, G., Pemán, J. and Sanchez-Reus, F. 2014. State of the art in the laboratory methods for the diagnosis of invasive fungal diseases. In microbiology for surgical infections, Elsevier, pp: 281–297. Ray, A., Aayilliath K.A., Banerjee, S., Chakrabarti, A. and Denning, D.W. 2022. Burden of serious fungal infections in India. Open Forum Infect. Dis. 9(12), ofac603. Ramana KV, Kandi S, Bharatkumar P.V, Sharada CH.V, Rao R, Mani R, Rao S.D. 2013. Invasive fungal infections: a comprehensive review. Am. J. Infect. Dis. Microbiol. 1(4), 64–69. Sehgal, I.S., Dhooria, S., Choudhary, H., Aggarwal, A.N., Garg, M., Chakrabarti, A. and Agarwal, R. 2019. Utility of serum and bronchoalveolar lavage fluid galactomannan in diagnosis of chronic pulmonary aspergillosis. J. Clin. Microbiol. 57(3), e01821–18. Sigera, L.S.M. and Denning, D.W. 2023. Invasive aspergillosis after renal transplantation. J. Fungi. 9(2), 255. Steinbach, W.J. 2012. Aspergillus species. In Principles and practice of pediatric infectious diseases, Eds., Long SS, Prober CG, Fischer M and Kimberlin D. Elsevier, pp: 1203–1209. Stynen, D., Sarfati, J., Goris, A., Prévost, M.C., Lesourd, M., Kamphuis, H., Darras, V. and Latgé, J.P. 1992. Rat monoclonal antibodies against Aspergillus galactomannan. Infect. Immun. 60(6), 2237–2245. Sun, W.K., Zhang, F., Xu, X.Y., Shen, Y.Y. and Shi, Y. 2010. A systematic review of the accuracy of diagnostic test of serum galactomannan antigen detection for invasive aspergillosis. Chinese J. Tubercul. Respir. Dis. 33(10), 758–765. Thornton, C.R. 2010. Detection of invasive aspergillosis. Adv. Appl. Microbiol. 70:187–216. Wu, Z., Wang, L., Tan, L., Wu, J., Chen, Z. and Hu, M. 2021. Diagnostic value of galactomannan in serum and bronchoalveolar lavage fluid for invasive pulmonary aspergillosis in non-neutropenic patients. Diagnost. Microbiol. Infect. Dis. 99(4), 115274. Xess, I., Pagano, L. and Dabas, Y. 2022. Invasive fungal infections 2021. J. Fungi. 8(8), 760. Zhou, W., Li, H., Zhang, Y., Huang, M., He, Q., Li, P., Zhang, F., Shi, Y. and Su, X. 2017. Diagnostic value of galactomannan antigen test in serum and bronchoalveolar lavage fluid samples from patients with nonneutropenic invasive pulmonary aspergillosis. J. Clin. Microbiol. 55(7), 2153–2161. | ||
| How to Cite this Article |
| Pubmed Style Choudhuri N, Chaudhuri BN, Guchhait P, Das A, Das S. Diagnostic significance of bronchoalveolar lavage galactomannan assay in invasive aspergillosis: A comprehensive analysis. J Microbiol Infect Dis. 2024; 14(1): 21 -29 . doi:10.5455/JMID.2024.v14.i1.4 Web Style Choudhuri N, Chaudhuri BN, Guchhait P, Das A, Das S. Diagnostic significance of bronchoalveolar lavage galactomannan assay in invasive aspergillosis: A comprehensive analysis. https://www.jmidonline.org/?mno=180327 [Access: January 23, 2026]. doi:10.5455/JMID.2024.v14.i1.4 AMA (American Medical Association) Style Choudhuri N, Chaudhuri BN, Guchhait P, Das A, Das S. Diagnostic significance of bronchoalveolar lavage galactomannan assay in invasive aspergillosis: A comprehensive analysis. J Microbiol Infect Dis. 2024; 14(1): 21 -29 . doi:10.5455/JMID.2024.v14.i1.4 Vancouver/ICMJE Style Choudhuri N, Chaudhuri BN, Guchhait P, Das A, Das S. Diagnostic significance of bronchoalveolar lavage galactomannan assay in invasive aspergillosis: A comprehensive analysis. J Microbiol Infect Dis. (2024), [cited January 23, 2026]; 14(1): 21 -29 . doi:10.5455/JMID.2024.v14.i1.4 Harvard Style Choudhuri, N., Chaudhuri, . B. N., Guchhait, . P., Das, . A. & Das, . S. (2024) Diagnostic significance of bronchoalveolar lavage galactomannan assay in invasive aspergillosis: A comprehensive analysis. J Microbiol Infect Dis, 14 (1), 21 -29 . doi:10.5455/JMID.2024.v14.i1.4 Turabian Style Choudhuri, Nairita, Bhaskar Narayan Chaudhuri, Partha Guchhait, Anupam Das, and Satadal Das. 2024. Diagnostic significance of bronchoalveolar lavage galactomannan assay in invasive aspergillosis: A comprehensive analysis. Journal of Microbiology and Infectious Diseases, 14 (1), 21 -29 . doi:10.5455/JMID.2024.v14.i1.4 Chicago Style Choudhuri, Nairita, Bhaskar Narayan Chaudhuri, Partha Guchhait, Anupam Das, and Satadal Das. "Diagnostic significance of bronchoalveolar lavage galactomannan assay in invasive aspergillosis: A comprehensive analysis." Journal of Microbiology and Infectious Diseases 14 (2024), 21 -29 . doi:10.5455/JMID.2024.v14.i1.4 MLA (The Modern Language Association) Style Choudhuri, Nairita, Bhaskar Narayan Chaudhuri, Partha Guchhait, Anupam Das, and Satadal Das. "Diagnostic significance of bronchoalveolar lavage galactomannan assay in invasive aspergillosis: A comprehensive analysis." Journal of Microbiology and Infectious Diseases 14.1 (2024), 21 -29 . Print. doi:10.5455/JMID.2024.v14.i1.4 APA (American Psychological Association) Style Choudhuri, N., Chaudhuri, . B. N., Guchhait, . P., Das, . A. & Das, . S. (2024) Diagnostic significance of bronchoalveolar lavage galactomannan assay in invasive aspergillosis: A comprehensive analysis. Journal of Microbiology and Infectious Diseases, 14 (1), 21 -29 . doi:10.5455/JMID.2024.v14.i1.4 |