| Case Report | ||
J Microbiol Infect Dis. 2023; 13(4): 200-203 J. Microbiol. Infect. Dis., (2023), Vol. 13(4): 200–203 Case Report Streptococcus constellatus and sinusitis: A brain abscess case seriesAnmol Johal1*, Sarah Esposito2, Ndausung Udongwo1, Benedict Cu1, Bhavroop Riar2, Walid Abboud1, Jose Fune3 and Edward Liu31Department of Internal Medicine, Jersey Shore University Medical Center, Neptune City, NJ 07753, USA 2School of Medicine, Saint George’s University, Grenada, USA 3Department of Infectious Disease, Jersey Shore University Medical Center, Neptune City, NJ 07753, USA *Corresponding Author: Anmol Johal. Department of Internal Medicine, Jersey Shore University Medical Center, Neptune, NJ. Email: anmol.johal [at] hmhn.org Submitted: 31/07/2023 Accepted: 14/12/2023 Published: 31/12/2023 © 2023 Journal of Microbiology and Infectious Diseases
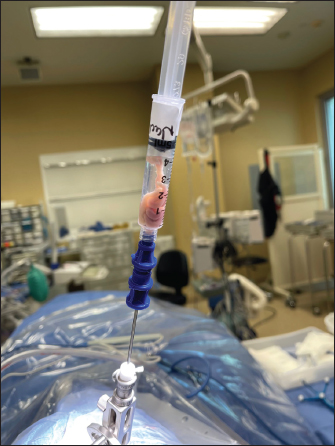
AbstractBackground: Brain abscess is an infectious process affecting the central nervous system and related structures and is many times the result of bacterial translocation from other areas of the body. Patients can present in a multitude of ways, ranging from sepsis to stroke, creating an ambiguous initial disease presentation. Through imaging, laboratory work, and surgical intervention and biopsy, an official diagnosis of brain abscess can be made; moreover, timely recognition and antibiotic treatment are paramount to creating positive patient outcomes. Case Description: We present a rare case of brain abscess caused by Streptococcus constellatus and Parvimonas micra from likely sinusitis, with appropriate biopsy and treatment. We aim to explore the underlying pathophysiology of brain abscesses, the different bacteria that may cause this disease process, differentials, and treatment. We also conducted a literature review for similar cases caused by Streptococcus constellatus and P. micra to highlight the rare yet dangerous nature of this infectious disease. Through our case report and series of similar cases, we presented the unusual development of brain abscess after a sinus infection and contiguous spread. Streptococcus constellatus and P. micra is an organism that can cause brain abscesses but is rare to be seen in individuals without sinus or odontogenic disease. Prompt identification through clinical signs, laboratories, imaging, cultures, and biopsies can help aid in creating better patient outcomes. Conclusion: This case series delineated the pathophysiology, diagnosis, and management of brain abscesses that resulted from contiguous bacterial spread. Timely diagnosis and treatment initiation are paramount to reducing mortality associated with brain abscesses. Keywords: Brain abscess, Infection, Sinusitis, Biopsy, Streptococcus. IntroductionBrain abscesses are rare but potentially fatal infections that can present with a variety of clinical manifestations and neurological deficits (Brouwer et al., 2014a,b). Brain abscesses are intracranial collections of purulent material that initially begin as localized areas of cerebral inflammation and can evolve into collections of pus and capsules (Muzumdar et al., 2011). Bacterial abscesses are most commonly caused by Streptococcus, Staphylococcus, and Enterobacteriaceae organisms (Brouwer et al., 2014a,b). There are many predisposing factors for brain abscess including contiguous infection, trauma, and hematogenous spread (Brook, 2017). Sinusitis as a cause of an intracranial abscess has been reported in the literature as being a rare yet dangerous complication of a sinus infection (Brook, 2005). Intracranial infections can range from abscesses and empyema to meningitis and osteomyelitis (Niehaus et al., 2018). The frontal lobe of the brain is the most common anatomical site for abscess formation and the majority of infections follow recent sinusitis or mastoiditis (Roche et al., 2003). Despite the high morbidity and mortality associated with this disease, early detection, surgical intervention, and antibiotic treatment can help better patient outcomes (Moorthy and Rajshekhar, 2008). Case DetailsA 51-year-old female with a past medical history significant for hypertension, hyperlipidemia, Graves’ disease, type II diabetes mellitus, aortic thrombosis s/p endovascular aneurysm repair and left lower extremity embolectomy, recurrent cerebrovascular accident, peripheral artery disease, history of deep vein thrombosis and pulmonary embolism on apixaban, chronic obstructive pulmonary disease, gastroesophageal reflux disease, and restless leg syndrome who presented to the emergency department with left-sided face, arm, and leg numbness and tingling and right-hand numbness. She endorsed that numbness started in her left thumb, index, and middle fingers 7 days before radiation to the left side of her body. She also endorsed left lower and upper extremity weakness, ataxia, and headache. On physical examination, the patient had left upper extremity pronator drift and 4/5 strength, ataxia on the finger to nose for the right upper extremity, and paresthesia to the left face, tongue, left upper and lower extremity as well as thumb, index, and middle fingers bilaterally. Dentition was normal with no prior history of odontogenic disease. Laboratories were significant for anemia with hemoglobin 11.6 g/dl (Ref. range 12.0–16.0 g/dl), blood urea nitrogen 26 mg/dl (Ref. range 9–23 mg/dl), acute kidney injury with creatinine 1.30 mg/dl (Ref. range 0.55–1.02 mg/dl), estimated glomerular filtration rate 43 (Ref. range >=60), hypernatremia 134 mmol/l (Ref. range 136–145 mmol/l), hypokalemia 3.0 (Ref. range 3.5–5.1 mmol/l), and C-reactive protein (CRP) 1.22 mg/l (Ref. range 0–3.00 mg/l). Computed tomography (CT) of the head without contrast revealed an area of edema and swelling of the right thalamus. Subsequent magnetic resonance imaging (MRI) of the brain with and without contrast revealed T2 and diffusion hyperintense peripherally T2 hypointense lesion in the right thalamus with prominent surrounding vasogenic edema. Differential diagnoses at this time included an evolving parenchymal hematoma or recently hemorrhaged cavernoma versus neoplasm, hemorrhagic metastasis, and atypical inflammatory or infectious etiology such as an abscess. She was given 10 mg dexamethasone and apixaban and clopidogrel were held due to concern for hemorrhage. A few days into the admission, a rapid response was called for worsening headache and lethargy. Repeat CT head revealed that the lesion had increased in size with worsening edema extending into the right cerebral peduncle, mass effect on the body of the right lateral ventricle, further effacement of the third ventricle, and midline shift. She was transferred to the neuro-intensive care unit for further care. Neurosurgery was reconsulted at this time. The patient was scheduled for a stereotactic needle biopsy of the brain the following day. The intraoperative specimen produced frank purulent fluid and was sent for both tissue culture and pathology (Fig. 1). Infectious disease was consulted for brain abscess possibly secondary to sinus disease with previous CT showing sinusitis and fluid in the maxillary sinus. She denied any previous neurological surgery or odontogenic disease. She was then started on metronidazole and ceftriaxone. Gram stain and cultures came back positive for Gram-positive cocci in pairs and chains. Antibiotics were broadened to include vancomycin for empiric coverage and concern for methicillin resistant Staphylococcus aureus. Cultures grew Streptococcus constellatus and Parvimonas micra. Based on the sensitivities of cultures, antibiotics were then narrowed to penicillin G 4 million units IV every 4 hours for a total of 37 days. About 6 days later, she was medically stable for discharge to home with outpatient follow-up.
Fig. 1. Brain abscess biopsy. DiscussionBrain abscesses are rare but potentially fatal infections of the central nervous system, as seen in the patient presented above. They can spread directly from the head and neck, seen in relation to otitis media 5% of the time as well as mastoiditis, paranasal sinus infections (30%–50% of the time), frontal and ethmoidal sinuses, and dental infections (Lange et al., 2018). Other causes of direct inoculation such as trauma or recent neurosurgery can also yield a brain abscess. Septicemia and hematogenous spread can also be the culprit; most commonly, a lung infection, such as pneumonia, empyema, or as a sequelae of cystic fibrosis can cause a distant seeding into the central nervous system (Lange et al., 2018). Other common causes of infectious spreading occur secondary to skin, pelvic, and intra-abdominal infections (Lange et al., 2018). While studies vary, the incidence of brain abscesses is estimated to be 8% of all intracranial masses in developing countries and 1%–2% in Western countries (Osenbach and Loftus, 1992). Contributing factors include AIDS and the use of broad-spectrum antibiotics and steroids. On the other hand, vaccinated children have yielded a decreased rate of brain abscess formation in that age group. Most commonly, adult men <30 years old and children between the ages of 4–7 years old are affected (Shachor-Meyouhas et al., 2010). A brain abscess develops over time with the histopathology changing with its development and can be broken down into four stages. The first stage, which occurs over the first 1–4 days, can be referred to as cerebritis, with localized edema and vascular congestion (Muzumdar et al., 2011). The second stage, which spans infection days 4–10, is called late cerebritis. Thereafter, an early capsule formation occurs from days 11–14, with the fourth and final stage, called the capsule formation, occurring after 2 weeks time (Muzumdar et al., 2011). After 2–3 weeks, liquefaction and necrosis occur (Hassel et al., 2018). In the case of continuous spread from the face and neck, bacterial species such as Bacteroides, Peptostreptococcus, and Streptococcus are the most common culprits. The latter Strep species are most commonly seen in patients with cardiovascular anomalies, such as cyanotic heart disease and right-to-left shunts (Muzumdar et al., 2011). Streptococcus and Staphylococcus can also be seen in prior neurosurgical interventions; these species, in addition to Clostridium and Enterobactericea, are seen in open-head trauma (Muzumdar et al., 2011). Fungal infections, Toxoplasma and Pseudomonas are typically seen in immunocompromised patients. This population includes patients with HIV, transplant recipients, those on chemotherapeutics, and long-term steroid usage (Muzumdar et al., 2011). History and physical provide key features that can help lead to correct diagnostic work-up; however, the nonspecific features can yield delayed treatment. Some common associated signs and symptoms include a headache, a change in mental status, focal neurological deficits, pain on the side where the abscess is located, fever, nausea, and vomiting (Patel and Clifford, 2014). The triad of headache, fever, and neurological deficit is seen in less than ⅓ of all reported cases, making it difficult to find pathognomonic signs of such a diagnosis (Patel and Clifford, 2014). Patient evaluation should include multiple sources of information and collateral information. Routine laboratory work, such as a complete blood count with differential, erythrocyte sedimentation rate, CRP, and blood cultures before the usage of antibiotics should be the initial laboratory work performed. As for imaging, an MRI is considered the modality of choice for diagnosis (Corsini Campioli et al., 2021). It allows radiologists and physicians to have a better perspective regarding the amount of inflammation and can detect satellite lesions otherwise missed by other imaging modalities (Corsini Campioli et al., 2021). However, a CT scan helps with early detection, and localization, and can show associated factors such as edema and elevated mass-associated midline shift (Muzumdar et al., 2011). Once identified, surgical aspiration can be utilized to culture the abscess for proper antibiotic and/or antifungal coverage (Corsini Campioli et al., 2021). Treatment regimens depend on the underlying pathogen causing the abscess. Gram-positive bacteria, as seen in our patient described above, can be covered with a third-generation cephalosporin or Penicillin G (as was used with our patient) (Hakan, 2008). Staph aureus and other Staph species such as Staph epidermidis should be covered with vancomycin; however, in the case of resistance, linezolid, trimethoprim-sulfamethoxazole, or daptomycin can be utilized (Hakan, 2008). Fungal infections are typically covered by Amphotericin B. Toxoplasmosis gondii is typically treated with pyrimethamine and sulfadiazine. In the case of Aspergillus, voriconazole can also be considered (Hakan, 2008). Surgical intervention can provide benefit on a case-by-case basis; these interventions can include craniotomies, burr holes, and CT-guided stereotactic procedures (Hakan, 2008). Intrathecal methods of antibiotic dosing can be utilized effectively as well (Nau et al., 2020). Other cases of brain abscesses have been reported in the literature and help to identify an outline of the patient’s presentation in an effort to provide better understanding and education on these diagnoses. One case documented a 69-year-old woman who had suffered a fall and presented to the emergency department with some concerning cerebellar signs on physical examination, including a lack of balance, wide-based gait, and a positive Romberg test (Carretero, 2017). Initial CT imaging suggested an acute ischemic infarction of the left cerebellar hemisphere; follow-up MRI imaging showed two cerebellar lesions that were hypointense in T1 and hyperintense in T2 with surrounding edema, findings consistent with a cerebellar abscess. Treatment with ceftriaxone and metronidazole was initiated with blood cultures yielding S. constellatus. Unfortunately, this patient passed away in the intensive care unit with later findings showing a pulmonary embolism (Carretero, 2017). Another case study of an otherwise healthy 48-year-old man who presented with a triad of fever, neurological deficit (right-sided weakness), and chills was published in 2018 (Mo et al., 2018). This patient had a similar work-up and was found to have an infection of Prevotella intermedia and S. constellatus. This patient abandoned treatment after 4 days and left the hospital despite their critical condition (Mo et al., 2018). A third study published in 2016 identified a 25-year-old male who had a rare left-sided thalamic abscess secondary to shrapnel injury (Senol et al., 2016). A comprehensive history and physical examination, alongside imaging and biopsy evaluations, showed that the patient had a brain abscess caused by Streptococcus constellatus (Senol et al., 2016). All of these studies within the literature show that S. constellatus is a common cause of brain abscesses. This bacteria is considered normal flora in the body, primarily within the oral cavity, urogenital region, and intestinal tract. When under a period of stress, such as during illness or immunocompromisation, this bacteria can form pyogenic abscesses in numerous places of the body. Other case studies have documented infections such as mediastinitis, pulmonary empyemas, pericarditis, and even abscesses of the liver. ConclusionThis case demonstrates a patient presentation and complete work-up that yielded the correct diagnosis of a brain abscess secondary to sinus disease with the utilization of biopsy and appropriate antibiotics that allowed for a favorable outcome. This case, along with other causes presented in the literature, delineates that a brain abscess is a diagnosis that needs to be made in a time-sensitive manner for an increased likelihood of a positive outcome. More case studies and literature reviews need to be performed to provide information about brain abscesses in the hopes of properly identifying them earlier and decreasing morbidity and mortality. AcknowledgmentNone. Statements and declarationsNone. ReferencesBrook, I. 2005. Microbiology of intracranial abscesses and their associated sinusitis. Arch. Otolaryngol. Head. Neck. Surg. 131(11), 1017–1019; doi:10.1001/archotol.131.11.1017 Brook, I. 2017. Microbiology and treatment of brain abscess. J. Clin. Neurosci. 38, 8–12; doi:10.1016/j.jocn.2016.12.035 Brouwer, M.C., Coutinho, J.M. and van de Beek, D. 2014. Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology 82(9), 806–813; doi:10.1212/WNL.0000000000000172 Brouwer, M.C., Tunkel, A.R., McKhann, G.M. 2nd. and van de Beek, D. 2014. Brain abscess. N. Engl. J. Med. 371(5), 447–456; doi:10.1056/NEJMra1301635 Carretero, R.G. 2017. Cerebellar abscesses, infective endocarditis and bacteremia due to a rare pathogen: Streptococcus constellatus. BMJ. Case. Rep. 2017, bcr-2017; doi: 10.1136/bcr-2017-221374 Corsini Campioli, C., Castillo Almeida, N.E., O’Horo, J.C., Garrigos, Z.E., Wilson, W.R., Cano, E., DeSimone, D.C., Baddour, L.M., Van Gompel, J.J. and Sohail, M.R. 2021. Bacterial brain abscess: an outline for diagnosis and management. Am. J. Med. 134(10), 1210–1217.e2; doi:10.1016/j.amjmed.2021.05.027 Hakan, T. 2008. Management of bacterial brain abscesses. Neurosurg. Focus. 24(6), E4; doi:10.3171/FOC/2008/24/6/E4 Hassel, B., De Souza, G.A., Stensland, M.E., Ivanovic, J., Voie, Ø. and Dahlberg, D. 2018. The proteome of pus from human brain abscesses: host-derived neurotoxic proteins and the cell-type diversity of CNS pus. J. Neurosurg. 129(3), 829–837; doi:10.3171/2017.4.JNS17284 Lange, N., Berndt, M., Jörger, A.K., Wagner, A., Wantia, N., Lummel, N., Ryang, Y.M., Meyer, B. and Gempt, J. 2018. Clinical characteristics and course of primary brain abscess. Acta. Neurochir. (Wien). 160(10), 2055–2062; doi:10.1007/s00701-018-3633-6 Mo, S., Wei, L., Chen, H., Li, R., Li, S. and Luo, G. 2018. A chinese case of Prevotella intermedia and Streptococcus constellatus intracranial mixed infection. Metab. Brain. Dis. 33(1), 161–166; doi: 10.1007/s11011-017-0142-x Moorthy, R.K. and Rajshekhar, V. 2008. Management of brain abscess: an overview. Neurosurg. Focus. 24(6), E3; doi:10.3171/FOC/2008/24/6/E3 Muzumdar, D., Jhawar, S. and Goel, A. 2011. Brain abscess: an overview. Int. J. Surg. 9(2), 136–144; doi:10.1016/j.ijsu.2010.11.005 Nau, R., Blei, C. and Eiffert H. 2020. Intrathecal antibacterial and antifungal therapies. Clin. Microbiol. Rev. 33(3), e00190–e00119; doi:10.1128/CMR.00190-19 Niehaus, M.T., Krape, K.N., Quinn, S.M. and Kane, B.G. 2018. Frontal sinusitis complicated by a brain abscess and subdural empyema. Radiol. Case. Rep. 13(2), 456–459; doi:10.1016/j.radcr.2018.02.003 Osenbach, R.K. and Loftus, C.M. 1992. Diagnosis and management of brain abscess. Neurosurg. Clin. N. Am. 3(2), 403–420. Patel, K. and Clifford, D.B. 2014. Bacterial brain abscess. Neurohospitalist 4(4), 196–204; doi:10.1177/1941874414540684 Roche, M., Humphreys, H., Smyth, E., Phillips, J., Cunney, R., McNamara, E., O’Brien, D. and McArdle, O. 2003. A twelve-year review of central nervous system bacterial abscesses; presentation and aetiology. Clin. Microbiol. Infect. 9(8), 803–809; doi:10.1046/j.1469-0691.2003.00651.x Senol, O., Suslu, H.T., Tetarli, N., Tiryaki, M. and Geclu, B. 2016. Thalamic abscess caused by a rare pathogen: Streptococcus constellatus. Pan. Afr. Med. J. 24, 256; doi: 10.11604/pamj.2016.24.256.9468 Shachor-Meyouhas, Y., Bar-Joseph, G., Guilburd, J.N., Lorber, A., Hadash, A. and Kassis, I. 2010. Brain abscess in children—epidemiology, predisposing factors and management in the modern medicine era. Acta. Paediatr. 99(8), 1163–1167; doi:10.1111/j.1651-2227.2010.01780.x | ||
| How to Cite this Article |
| Pubmed Style Johal AS, Esposito S, Udongwo N, Cu B, Riar BK, Abboud W, Fune J, Liu E. Streptococcus constellatus and sinusitis: A brain abscess case series. J Microbiol Infect Dis. 2023; 13(4): 200-203. doi:10.5455/JMID.2023.v13.i4.6 Web Style Johal AS, Esposito S, Udongwo N, Cu B, Riar BK, Abboud W, Fune J, Liu E. Streptococcus constellatus and sinusitis: A brain abscess case series. https://www.jmidonline.org/?mno=163175 [Access: January 25, 2026]. doi:10.5455/JMID.2023.v13.i4.6 AMA (American Medical Association) Style Johal AS, Esposito S, Udongwo N, Cu B, Riar BK, Abboud W, Fune J, Liu E. Streptococcus constellatus and sinusitis: A brain abscess case series. J Microbiol Infect Dis. 2023; 13(4): 200-203. doi:10.5455/JMID.2023.v13.i4.6 Vancouver/ICMJE Style Johal AS, Esposito S, Udongwo N, Cu B, Riar BK, Abboud W, Fune J, Liu E. Streptococcus constellatus and sinusitis: A brain abscess case series. J Microbiol Infect Dis. (2023), [cited January 25, 2026]; 13(4): 200-203. doi:10.5455/JMID.2023.v13.i4.6 Harvard Style Johal, A. S., Esposito, . S., Udongwo, . N., Cu, . B., Riar, . B. K., Abboud, . W., Fune, . J. & Liu, . E. (2023) Streptococcus constellatus and sinusitis: A brain abscess case series. J Microbiol Infect Dis, 13 (4), 200-203. doi:10.5455/JMID.2023.v13.i4.6 Turabian Style Johal, Anmol Singh, Sarah Esposito, Ndausung Udongwo, Benedict Cu, Bhavroop Kaur Riar, Walid Abboud, Jose Fune, and Edward Liu. 2023. Streptococcus constellatus and sinusitis: A brain abscess case series. Journal of Microbiology and Infectious Diseases, 13 (4), 200-203. doi:10.5455/JMID.2023.v13.i4.6 Chicago Style Johal, Anmol Singh, Sarah Esposito, Ndausung Udongwo, Benedict Cu, Bhavroop Kaur Riar, Walid Abboud, Jose Fune, and Edward Liu. "Streptococcus constellatus and sinusitis: A brain abscess case series." Journal of Microbiology and Infectious Diseases 13 (2023), 200-203. doi:10.5455/JMID.2023.v13.i4.6 MLA (The Modern Language Association) Style Johal, Anmol Singh, Sarah Esposito, Ndausung Udongwo, Benedict Cu, Bhavroop Kaur Riar, Walid Abboud, Jose Fune, and Edward Liu. "Streptococcus constellatus and sinusitis: A brain abscess case series." Journal of Microbiology and Infectious Diseases 13.4 (2023), 200-203. Print. doi:10.5455/JMID.2023.v13.i4.6 APA (American Psychological Association) Style Johal, A. S., Esposito, . S., Udongwo, . N., Cu, . B., Riar, . B. K., Abboud, . W., Fune, . J. & Liu, . E. (2023) Streptococcus constellatus and sinusitis: A brain abscess case series. Journal of Microbiology and Infectious Diseases, 13 (4), 200-203. doi:10.5455/JMID.2023.v13.i4.6 |