| Research Article | ||
J. Microbiol. Infect. Dis., (2024), Vol. 14(1): 13–20 Original Research Effect of the COVID-19 epidemic on drug resistance of Staphylococcus aureus infectionChaohui Zhao1†, Zhaowang Guo2†, Xinghua Li1, Yuxin Ji1, Tingting Li1, Meiyi Li1, Chengzhuo Wang1, Jie Chen1, Yuting Luo1 and Xi Liu1*1Department of Infectious Diseases, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, China 2Clinical Laboratory, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, China †Both authors contributed equally to this work *Corresponding Author: Xi Liu. Department of Infectious Diseases, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, China. Email: liuxi26 [at] mail.sysu.edu.cn Submitted: 12/10/2023 Accepted: 24/03/2024 Published: 31/03/2024 © 2024 Journal of Microbiology and Infectious Diseases
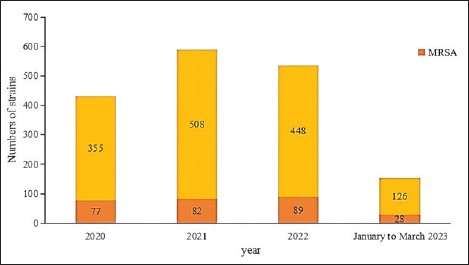
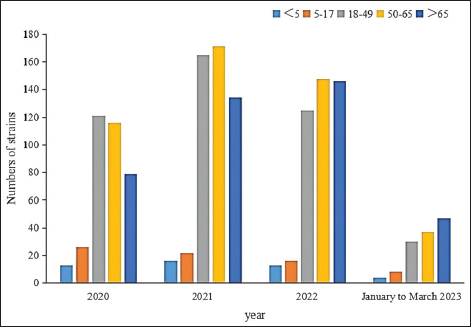
AbstractIntroduction: Staphylococcus aureus was associated with more than 1 million deaths in 2019. The coronavirus disease 2019 (COVID-19) pandemic has lasted three years. In China, measures to control COVID-19 cases were adjusted in December 2022. In this study, we investigated the effects of the COVID-19 pandemic on the drug resistance of S. aureus infection. Aim: Understanding the impact of the adjustment of COVID-19 control measures on the drug sensitivity of S. aureus will provide a reference for empirical drug use and the prevention of clinical S. aureus infection. Methods: A retrospective analysis was performed, focusing on the clinical distribution and drug resistance of distributions of S. aureus isolated from the Fifth Affiliated Hospital of Sun Yat-sen University during the COVID-19 pandemic from January 2020 to March 2023. The effects of COVID-19 control measures on the drug resistance of S. aureus infection were further investigated. Results: A total of 1,437 strains of S. aureus were isolated, with a methicillin-resistant S. aureus detection rate of 19.2%. The department with the highest detection rate was the oncology department (17.0%, 244/1,437), followed by the intensive care unit (11.1%, 159/1,437), the dermatology department (10.7%, 154/1,437), and the wound repair and burn department (9.7%, 139/1,437). The most frequent specimens were secretions (42.0%, 603/1,437), followed by sputum (23.3%, 335/1,437), blood (10.7%, 154/1,437), and pus (5.4%, 78/1,437). The population distribution was primarily male (62.3%, 895/1,437). S. aureus infections were most common among those aged 50–65 (32.8%, 472/1,437), followed by those aged 18–49 (30.7%, 441/1,437). Staphylococcus aureus was most resistant to penicillin G (86.3%), followed by erythromycin (37.9%) and clindamycin (37.3%). The antibiotics with no resistance were linezolid, teicoplanin, tigecycline, and vancomycin. There was no significant difference in the drug-resistance rate between COVID-19–positive and COVID-19–negative patients. Conclusion: The outbreak of COVID-19 in 2020 and the adjustment of control measures had no significant impact on the drug resistance of S. aureus infection. Keywords: Clinical distribution, COVID-19 infection, Drug-resistance characteristics, Staphylococcus aureus. IntroductionInfectious diseases are major diseases that cause death worldwide and led to an estimated 13.7 million infection-related deaths in 2019 (GBD 2019 Antimicrobial Resistance Collaborators, 2022). Staphylococcus aureus widely exists in nature, is the most common clinical gram-positive pathogen (Hu et al., 2022), and is also one of the main pathogens causing community and hospital-acquired infections (Yin et al., 2018). Because it carries virulence genes, it can produce a variety of virulence factors, causing damage to host cells and triggering resistance to the host immune system (Atshan et al., 2021), and it has a wide range of both pathogenicity and antimicrobial resistance. Staphylococcus aureus is an invasive pathogen that can cause infections in different tissues and parts of the body (Zong et al., 2021). In recent years, the abuse of antibiotics has led to an increase in bacterial resistance and the emergence of multidrug-resistant bacteria, which not only increases the economic burden on patients and prolongs hospital stays (Xu et al., 2017) but also poses great challenges to clinical treatment. Staphylococcus aureus, as a common clinical pathogen, is the main pathogen causing death in bacterial infections and one of the main pathogens causing increased economic burden; thus, studying the clinical distribution and drug-resistance characteristics of S. aureus is of great significance. This paper aims to study the clinical distribution of S. aureus during the coronavirus disease 2019 (COVID-19) pandemic from January 2022 to March 2023. From January 2020 to December 2022, China implemented different measures for combatting COVID-19—including containment and control, control of personnel flow, isolation of COVID-19–positive patients and close contacts, avoidance of large gatherings, strengthening community management, improving public health awareness, and large-scale promotion of personal protective equipment—such that the epidemic situation in China was well controlled. After December 2022, however, these measures were lifted, and work and production resumed. Recently, the number of COVID-19–positive patients has increased day by day. Hospitals across China have since received COVID-19–positive patients on a large scale, and 3,291 COVID-19–positive patients were received by our hospital between December 25, 2022, and February 14, 2023. Understanding the impact of the adjustment of COVID-19 control measures on the drug sensitivity of S. aureus in December 2022 will provide a reference for empirical drug use and the prevention of clinical S. aureus infection. Materials and MethodsSource of materialsA retrospective analysis of S. aureus strains isolated from the Fifth Affiliated Hospital of Sun Yat-sen University from January 2020 to March 2023 was performed. Study exclusion criteria included the following: (1) incomplete clinical data; (2) two or more pathogenic bacteria were simultaneously isolated from the same specimen; (3) when multiple strains were isolated from the same patient within 1 month, they were considered to belong to the same strain, and the first isolated strain was used as a representative strain; and (4) there are no drug sensitivity results. After applying the exclusion criteria, a total of 1,437 strains were selected for inclusion in this study. We collected information on the age, sex, inpatient department, specimen type, and drug sensitivity of the patients. MethodsFor bacterial identification and drug sensitivity testing, the samples were inoculated and incubated in a blood plate for 18–24 h. Suspected colonies were selected and coated with a target plate, and matrix solution was added to dry them before the colonies and target plate were placed into a MALDI-TOF MS mass spectrometer (Merieux, France) for identification. Then, 280 µl (0.5–0.67 of McDonnell’s bacterial suspension) of the bacteria to be identified was taken and mixed with 3 ml of physiological saline. The Chinese custom card GP639 was loaded into the vitek-2 compact fully automatic drug sensitivity analyzer for drug-sensitivity testing. The quality control strain was ATCC29213 S. aureus (American Type Culture Collection, Manassas, VA). Statistical analysisSPSS version 26.0 (IBM Corporation, Armonk, NY) was used for data analysis, with counting data expressed as a percentage (%). Differences in rates between groups were analyzed using a two-tailed test or Fisher’s exact test. At the bilateral test level, α=0.05, and the difference was statistically significant when p < 0.05. Ethical approvalThis study involving human participants was reviewed and approved by the institutional review board of the Fifth Affiliated Hospital of Sun Yat-sen University (Zhuhai, China) (no. ZDWY [2023] Lunzi No. [K98-1]). All patients provided their written informed consent to participate in the study. ResultsIsolation of S. aureus according to timeThe highest number of strains was isolated in 2021 (n=508 strains), while 355 strains were isolated in 2020, 448 strains were isolated in 2022, and 126 strains were isolated from January to March 2023. Among them, the incidence of methicillin-resistant S. aureus (MRSA) ranged from 16.1%–22.2% (p=0.148). The annual isolation amounts and MRSA isolation rates of S. aureus are shown in Figure 1.
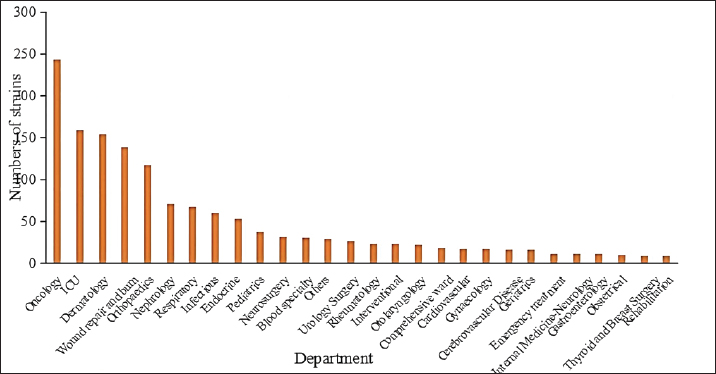
Fig. 1. Staphylococcus aureus isolates identified annually during 2020 to March 2023. Departmental distribution of isolated strainsThe department with the highest rate of S. aureus detection was the oncology department (17.0%, 244/1,437), followed by the intensive care unit (11.1%, 159/1,437), the dermatology department (10.7%, 154/1,437), the wound repair and burn department (9.7%, 139/1,437), the orthopedics department (8.2%, 118/1,437), the nephrology department (4.9%, 71/1,437), the respiratory department (4.7%, 67/1,437), the infectious disease department (4.2%, 60/1,437), and other departments. The distribution of departments infected with S. aureus is shown in Figure 2.
Fig. 2. Distribution of departments in patients with S. aureus infection. Note: “Other departments” include cardiothoracic surgery, hepatobiliary surgery, gastrointestinal surgery, general practice, ophthalmology, the COVID-19 ward, stomatology, the fever clinic, and the provincial famous medical expert clinic.
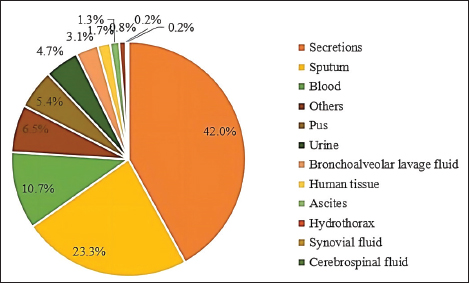
Fig. 3. Distribution of S. aureus specimens.
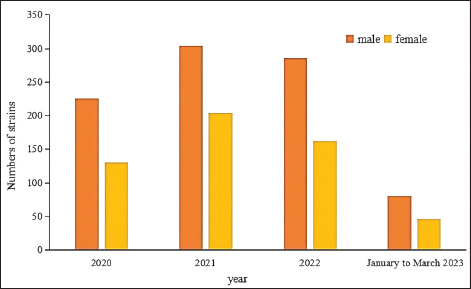
Fig. 4. Sex distribution of the study population.
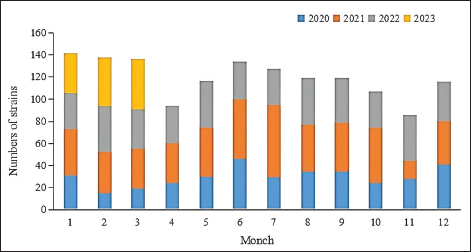
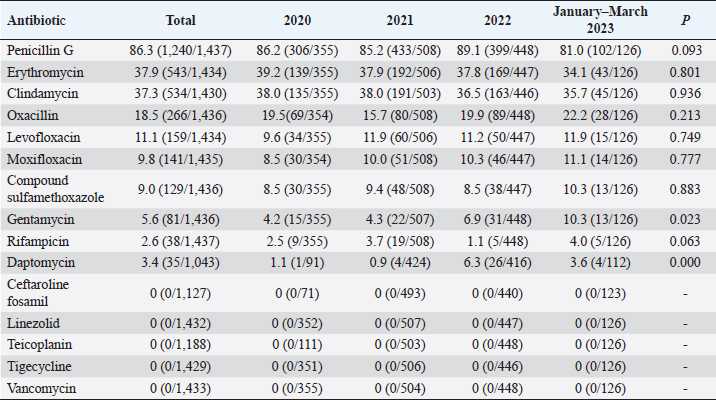
Fig. 5. Age distribution of the study population. Distribution of S. aureus specimensThe highest isolation rate of S. aureus specimens was found in secretions (42.0%, 603/1,437), followed by sputum (23.3%, 335/1,437), blood (10.7%, 154/1,437), pus (5.4%, 78/1,437), urine (4.7%, 68/1,437), pulmonary lavage fluid (3.1%, 44/1,437), and human tissue (1.7%, 25/1,437). The distribution of S. aureus specimens is shown in Figure 3. Distribution of S. aureus populationRegarding sex distribution among infected patients, male patients accounted for 62.3% of infections (895/1,437) and female patients accounted for 37.7% of infections (542/1,437), as shown in Figure 4. Regarding age distribution, the greatest proportion of S. aureus infections was among patients aged 50–65 (32.8%, 472/1,437), followed by those aged 18–49 (30.7%, 441/1,437) and those aged >65 (28.3%, 406/1,437), as shown in Figure 5. Time variation of S. aureus isolatesThe detection rate of S. aureus varied between months, with the highest detection rates in different years documented in June 2020, July 2021, May 2022, and March 2023, respectively, as shown in Figure 6. Drug-resistance characteristics of S. aureusThe highest resistance rate of S. aureus was 86.3% (to penicillin G), followed by 37.9% (erythromycin), 37.3% (clindamycin), 18.5% (oxacillin), 11.1% (levofloxacin), 9.8% (moxifloxacin), 9.0% (compound sulfamethoxazole), 5.6% (gentamycin), 2.6% (rifampicin), and 3.4% (daptomycin). No resistance to ceftaroline fosamil, linezolid, teicoplanin, tigecycline, or vancomycin was documented, as shown in Table 1. The change analysis of drug resistance results revealed that the drug-resistance rates of penicillin G, erythromycin, and clindamycin remained relatively stable and high (p > 0.05). Moreover, the rate of resistance to gentamicin has been increasing year by year (p=0.023), while the rate of resistance to daptomycin increased in 2022 compared to 2020 and 2021 but then decreased from January to March 2023 compared to 2022 (p=0.000).
Fig. 6. Time distribution of S. aureus isolation. Table 1. Resistance rate of S. aureus to antibiotics between 2020 and March 2023.
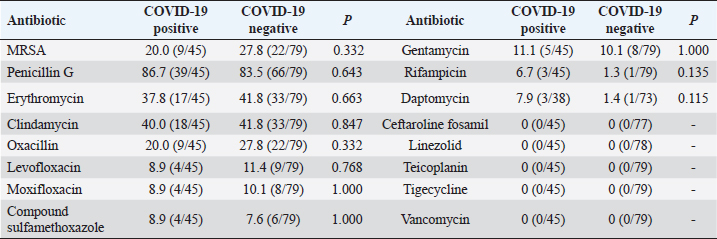
Comparison of drug resistance of S. aureus between COVID-19–positive patients and COVID-19–negative patientsDuring the period from December 1, 2022, to February 28, 2023, there were 124 patients with positive S. aureus cultures, of which COVID-19 patients accounted for 36.3% (45/124); then, during the period from December 25, 2022, to February 14, 2023, our hospital received 3,291 patients with COVID-19, including 38 patients with S. aureus infections, accounting for 1.2%. Overall, the MRSA detection rate was 20.0% among COVID-19–positive patients and 27.8% among COVID-19–negative patients (p=0.332). The rates of resistance to daptomycin and rifampicin among COVID-19–positive patients were slightly higher than those among COVID-19–negative patients, but there was no statistical difference (p > 0.05); conversely, there was no significant difference in the rates of resistance to penicillin G, erythromycin, clindamycin, levofloxacin, moxifloxacin, compound sulfamethoxazole, and gentamicin between COVID-19–positive patients and COVID-19–negative patients (p > 0.05), as shown in Table 2. Table 2. Rates of antibiotic resistance among COVID-19–positive and COVID-19–negative patients (%).
DiscussionStaphylococcus aureus is a highly invasive and pathogenic pathogen that can cause multiple infections and is widely distributed in various clinical departments. Research has shown that invasive procedures, such as placement of indwelling central venous catheters; prolonged hospital stays; combined use of antibiotics; hormone therapy; malignant tumors; burns; underlying diseases; and age >60 are high-risk factors for MRSA infection (Atmaca et al., 2014; Yasmin et al., 2016; Yang et al., 2016; Wang et al., 2022). Considering the distribution of isolates among departments, the oncology department has the highest detection rate in our hospital, followed by the intensive care unit and dermatology department, which may be related to factors such as the low immune function of tumor patients, long hospital stays, and persistence of deep vein catheters. The high infection rate of S. aureus in intensive care unit patients may be related to multiple underlying diseases as well as invasive procedures such as deep vein catheterization, tracheal intubation, and ventilator use. From June 2021 to May 2022, the infectious disease department focused on the treatment of COVID-19 patients, and the isolation rate of S. aureus was low. According to the distribution of samples, the majority of S. aureus isolates were found in secretions, followed by respiratory tract samples and blood samples. According to the distribution of samples and departments, the detection rate among dermatology patients in our hospital was high and the detection rate of S. aureus in secretion samples was high, which may be attributed to the possibility that the colonization rate of S. aureus in patients with purulent sweat glands/acne and other hair follicle skin diseases is higher than that of the general population (Katoulis et al., 2020). Therefore, as a general rule, the rate of infection with S. aureus is high. Studies have shown that S. aureus is the main pathogen causing skin and soft tissue infections (Abrahamian et al., 2019; Wan et al., 2019), which can cause suppurative infections of local tissues and organs. Therefore, the rate of isolating S. aureus from secretions and pus cultures was high, but it cannot be ruled out that some findings may be colonized bacteria. There are studies reporting that men secrete more oil due to the action of male hormones, and S. aureus is more prone to infect men than women (Olsen et al., 2012). However, in this study, male infections accounted for 62.3%, while female infections accounted for 37.7% (p > 0.05), with no statistically significant difference in the proportions of infections between men and women. In this study, the proportion of patients aged 50–65 infected with S. aureus was greatest, 32.8%, followed by those aged 18–49 (30.7%) and those aged >65 (28.3%) (p=0.009). The proportion of patients aged >65 in 2022 and January to March 2023 increased compared to the earlier level in the study, which may be related to the adjustment of COVID-19 control measures in December 2022, the obvious increase in COVID-19 inpatients, and the increase in the number of elderly inpatients. Some studies have shown that the rate of S. aureus infection increases with age(Chen et al., 2010), while others have shown that S. aureus infection is more common in young patients(Anderson et al., 2008). The age distribution and causes of S. aureus infection require further research. Based on the time distribution of this study, there is no fixed month displaying the highest detection rate of S. aureus. The isolation rate of MRSA in China is declining year by year. The detection rate of MRSA in China was 31.4% in 2019, 31.0% in 2020 (Hu et al., 2021), and 30% in 2021(Hu et al., 2022), respectively. Similarly, the detection rate of COVID-19 is still declining year by year since the pandemic began in 2020. The detection rate of MRSA in our hospital has been 19.2% since the COVID-19 pandemic began in 2020, which is lower than the national average; more specifically, the MRSA detection rate of COVID-19–negative patients were 27.8% and the MRSA detection rate of COVID-19–positive patients was 20% (p=0.332). In other words, COVID-19 diagnosis and the adjustment of control measures had no impact on the MRSA detection rate. Notably, the MRSA detection rate in our study region was lower than the national average. Staphylococcus aureus has a high degree of resistance to penicillin G, clindamycin, and erythromycin, which is consistent with the findings of previous studies (Yin et al., 2018; Kang et al., 2020). Among them, the penicillin resistance rate was the highest but still lower than the national average (Hu et al., 2022). In 2021, national bacterial resistance monitoring found that there were strains resistant to tigecycline. In this study, however, there was no resistance to linezolid, teicoplanin, tigecycline, or vancomycin detected. There was also no significant difference in drug resistance to penicillin, quinolone, and other drugs between COVID-19–positive patients and COVID-19–negative patients. Bacterial resistance is closely related to the use of antibiotics. The unreasonable use of antibiotics has made the problem of bacterial resistance increasingly severe. Although the rate of MRSA detection has shown a decreasing trend in recent years, the mortality rate caused by MRSA remains high, and MRSA can be transmitted in hospitals through various pathways such as wound contact. Reasonable use of antibiotics, strict hand hygiene, environmental disinfection, and contact isolation are important measures to reduce MRSA infection and transmission. The resistance of S. aureus is related to biofilm formation (Tang and Jin, 2022). In addition to the use of antibiotics, non-antibacterial treatment methods studied in recent years, such as phage therapy, photodynamic therapy, and biofilm-degrading enzyme therapy, which use S. aureus resistance–related substances as the entry point, may become alternative methods for antibiotic treatment. To summarize, the detection rate of MRSA in China is declining year by year. COVID-19 status and the adjustment of epidemic control measures had no significant impact on the drug resistance of S. aureus. The detection rate of MRSA and the drug-resistance rate in Zhuhai were lower than the national level, and the level of bacterial drug resistance may vary between different regions. When empirically selecting antibiotics to treat bacterial infections, antibiotics should be reasonably selected in combination with local bacterial drug resistance. AcknowledgmentThe authors thank Director Liu Xi for her full guidance in the research and revision of the article. They thank Dr. Guo Zaowang for helping to collect cultivation and drug sensitivity data and modify the article. They thank Dr. Li Xinghua for his quality control of the study. They also thank Drs. Ji Yuxin, Li Tingting, Li Meiyi, Wang Chengzhuo, Chen Jie, and Luo Yuting for helping to screen the enrolled cases. Conflict of interestThe authors declare that there is no conflict of interest. FundingNone. Data availabilityAll data are provided in the manuscript. Authors contributionsAll authors contributed to this study. All authors read and approved the final manuscript. ReferencesAbrahamian, F.M., Sakoulas, G., Tzanis, E., Manley, A., Steenbergen, J., Das, A.F., Eckburg, P.B. and McGovern, P.C. 2019. Omadacycline for acute bacterial skin and skin structure infections. Clin. Infect. Dis. 69, S23–S32. Anderson, D.J., Chen, L.F., Schmader, K.E., Sexton, D.J., Choi, Y., Link, K., Sloane, R. and Kaye, K.S. 2008. Poor functional status as a risk factor for surgical site infection due to methicillin-resistant Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 29, 832–839. Atmaca, Ö., Zarakolu, P., Karahan, C., ÇAkir, B. and ÜNal, S. 2014. Risk factors and antibiotic use in methicillin-resistant Staphylococcus aureus bacteremia in hospitalized patients at hacettepe university adult and oncology hospitals (2004–2011) and antimicrobial susceptibilities of the isolates: a nested case-control study. Microbiol. Bull. 48, 523–537. Atshan, S.S., Hamat, R.A., Coolen, M.J.L., Dykes, G., Sekawi, Z., Mullins, B.J., Than, L.T.L., Abduljalee, S.A. and Kicic, A. 2021.The role of subinhibitory concentrations of daptomycin and tigecycline in modulating virulence in Staphylococcus aureus. Antibiotics 10, 39. Chen, T.Y., Anderson, D.J., Chopra, T., Choi, Y., Schmader, K.E. and Kaye, K.S. 2010. Poor functional status is an independent predictor of surgical site infections due to methicillin-resistant Staphylococcus aureus in older adults. J. Am. Geriatr. Soc. 58, 527–532. GBD. 2019. Antimicrobial Resistance Collaborators. 2022. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 400, 2221–2248. Hu, F., Guo, Y., Zhu, D., Wang, F., Jiang, X., Xu, Y., Zhang, X., Zhang, Z., Ji, P., Xie, Y., Kang, M., Wang, C., Wang, A., Xu, Y., Huang, Y., Sun, Z., Chen, Z., Ni, Y., Sun, J., Chu, Y., Tian, S., Hu, Z., Li, J., Yu, Y., Lin, J., Shan, B., Du, Y., Guo, S., Wei, L., Zou, F., Zhang, H., Wang, C., Hu, Y., Ai, X., Zhuo, C., Su, D., Guo, D., Zhao, J., Yu, H., Huang, X., Liu, W., Li, Y., Jin, Y., Shao, C., Xu, X., Yan, C.,Wang, S., Chu, Y., Zhang, L., Ma, J., Zhou, S., Zhou, Y., Zhu, L., Meng, J., Dong, F., Zheng,H., Hu, F., Shen, H., Zhou, W., Jia,W., Li, G., Wu, J., Lu, Y., Li, J., Duan, J., Kang, J., Ma, X., Zheng, Y., Guo, R., Zhu, Y., Chen, Y., Meng, Q., Wang, S., Hu, X., Shen, J., Wang, R., Fang, H., Yu, B., Zhao, Y., Gong, P., Weng, K., Zhang, Y., Liu, J., Liao, L., Gu, H., Jiang, L., He, W., Xue, S., Feng, J., Dou, R. and Yue, C. 2021. CHINET surveillance of bacterial resistance: Results of 2020. Chin. J. Infect. Chemother. 21, 377–387. Hu, F., Guo, Y., Zhu, D., Wang, F., Jiang, X., Xu, Y., Zhang, X., Zhang, Z., Ji, P., Xie, Y., Kang, M., Wang, C., Wang, A., Xu, Y., Huang, Y., Sun, Z., Chen, Z., Ni, Y., Sun, J., Chu, Y., Tian, S., Hu, Z., Li, J., Yu, Y., Lin, J., Shan, B., Du, Y., Guo, S., Wei, L., Zou, F., Zhang, H., Wang, C., Hu, Y., Ai, X., Zhuo, C., Su, D., Guo, D., Zhao, J., Yu, H., Huang, X., Liu, W., Li, Y., Jin, Y., Shao, C., Xu, X., Yan, C.,Wang, S., Chu, Y., Zhang, L., Ma, J., Zhou, S., Zhou, Y., Zhu, L., Meng, J., Dong, F., Lü, Z., Hu, F., Shen, H., Zhou, W., Jia, W., Li, G., Wu, J., Lu, Y., Li, J., Duan, J., Kang, J., Ma, X., Zheng, Y., Guo, R., Zhu, Y., Chen, Y., Meng, Q., Wang, S., Hu, X., Shen, J., Wang, R., Fang, H., Yu, B., Zhao, Y., Gong, P., Weng, K., Zhang, Y., Liu, J., Liao, L., Gu, H., Jiang, L., He, W., Xue, S., Feng, J. and Yue, C. 2022. CHINET surveillance of antimicrobial resistance among the bacterial isolates in 2021. Chin. J. Infect. Chemother. 22, 521–530. Kang, Y.L. and Tian, J.J. 2020. Clinical study on the distribution characteristics and drug resistance of Staphylococcus aureus. Contemp. Med. Forum 18, 167–168. Katoulis, A., Koumaki, V., Efthymiou, O., Koumaki, D., Dimitroulia, E., Voudouri, M., Voudouri, A., Bozi, E. and Tsakris, A. 2020. Staphylococcus aureus carriage status in patients with hidradenitis suppurativa: an observational cohort study in a tertiary referral hospital in Athens, Greece. Dermatology 236, 31–36. Olsen, K., Falch, B.M., Danielsen, K., Johannessen, M., Ericson Sollid, J.U., Thune, I., Grimne, G., Jorde, R., Simonsen, G.S. and Furberg, A.-S. 2012. Staphylococcus aureus nasal carriage is associated with serum 25-hydroxyvitamin D levels, gender and smoking status. The Tromsø staph and skin study. Eur. J. Clin. Microbiol. Infect. Dis. 31, 465–473. Tang, L. and Jin, Y.L. 2022. New advances in prevention and treatment of Staphylococcus aureus infection. Prog. Microbiol. Immunol. 50, 66–71. Wan, D., Wan, Y., Zhu, H., Wang, F. and Hou, J. 2019. Prevalence of Staphylococcus aureus infection in patients with skin and soft tissue infection and resistance to linezolid and vancomycin. Chin. J. Nosocomiol. 29, 32–35. Wang, J., Dong, A., Xing, H., Li, N., Wang, N., Xu, Y., Guo, J. and Li, S. 2022. Analysis of infection factors and drug resistance of invasive methicillin-susceptible Staphylococcus aureus infection. Northwest Pharm. J. 37, 146–151. Xu, B., Yuan, H. and Yang, P. 2017. Evaluation of economic burden induced by multidrug-resistant bacteria related infectons in a tertiary general hospital. Chin. J. Exp. Clin. Infect. Dis. (Electron Ed) 11, 455–459. Yang, L. and Chen, H. 2016. Meta-analysis on risk factors for hospital-acquired methicillin-resistant Staphylococcus aureus infection in China. Int. J. Epidemiol. Infect. Dis. 43, 23–29. Yasmin, M., El Hage, H., Obeid, R., El Haddad, H., Zaarour, M. and Khalil, A. 2016. Epidemiology of bloodstream infections caused by methicillin-resistant Staphylococcus aureus at a tertiary care hospital in New York. Am. J. Infect. Control 44, 41–46. Yin, S., Wang, L. and Sheng, F. 2018. Analysis of antibiotic use and bacterial resistance in our hospital in 2016. Northwest Pharm. J. 33, 682–687. Zong, S., Xu, Y., Ding, X. and Xu, P. 2021. Characteristics of minimum inhibitory concentration drift of vancomycin against methicillin-resistant Staphylococcus aureus in Xuzhou in 2015–2019. Chin. J. Infect. Control 20, 991–995. | ||
| How to Cite this Article |
| Pubmed Style Zhao C, Guo Z, Li X, Ji Y, Li T, Li M, Wang C, Chen J, Luo Y, Liu X. Effect of the COVID-19 epidemic on drug resistance of Staphylococcus aureus infection. J Microbiol Infect Dis. 2024; 14(1): 13-20 . doi:10.5455/JMID.2024.v14.i1.3 Web Style Zhao C, Guo Z, Li X, Ji Y, Li T, Li M, Wang C, Chen J, Luo Y, Liu X. Effect of the COVID-19 epidemic on drug resistance of Staphylococcus aureus infection. https://www.jmidonline.org/?mno=161283 [Access: June 30, 2025]. doi:10.5455/JMID.2024.v14.i1.3 AMA (American Medical Association) Style Zhao C, Guo Z, Li X, Ji Y, Li T, Li M, Wang C, Chen J, Luo Y, Liu X. Effect of the COVID-19 epidemic on drug resistance of Staphylococcus aureus infection. J Microbiol Infect Dis. 2024; 14(1): 13-20 . doi:10.5455/JMID.2024.v14.i1.3 Vancouver/ICMJE Style Zhao C, Guo Z, Li X, Ji Y, Li T, Li M, Wang C, Chen J, Luo Y, Liu X. Effect of the COVID-19 epidemic on drug resistance of Staphylococcus aureus infection. J Microbiol Infect Dis. (2024), [cited June 30, 2025]; 14(1): 13-20 . doi:10.5455/JMID.2024.v14.i1.3 Harvard Style Zhao, C., Guo, . Z., Li, . X., Ji, . Y., Li, . T., Li, . M., Wang, . C., Chen, . J., Luo, . Y. & Liu, . X. (2024) Effect of the COVID-19 epidemic on drug resistance of Staphylococcus aureus infection. J Microbiol Infect Dis, 14 (1), 13-20 . doi:10.5455/JMID.2024.v14.i1.3 Turabian Style Zhao, Chaohui, Zhaowang Guo, Xinghua Li, Yuxin Ji, Tingting Li, Meiyi Li, Chengzhuo Wang, Jie Chen, Yuting Luo, and Xi Liu. 2024. Effect of the COVID-19 epidemic on drug resistance of Staphylococcus aureus infection. Journal of Microbiology and Infectious Diseases, 14 (1), 13-20 . doi:10.5455/JMID.2024.v14.i1.3 Chicago Style Zhao, Chaohui, Zhaowang Guo, Xinghua Li, Yuxin Ji, Tingting Li, Meiyi Li, Chengzhuo Wang, Jie Chen, Yuting Luo, and Xi Liu. "Effect of the COVID-19 epidemic on drug resistance of Staphylococcus aureus infection." Journal of Microbiology and Infectious Diseases 14 (2024), 13-20 . doi:10.5455/JMID.2024.v14.i1.3 MLA (The Modern Language Association) Style Zhao, Chaohui, Zhaowang Guo, Xinghua Li, Yuxin Ji, Tingting Li, Meiyi Li, Chengzhuo Wang, Jie Chen, Yuting Luo, and Xi Liu. "Effect of the COVID-19 epidemic on drug resistance of Staphylococcus aureus infection." Journal of Microbiology and Infectious Diseases 14.1 (2024), 13-20 . Print. doi:10.5455/JMID.2024.v14.i1.3 APA (American Psychological Association) Style Zhao, C., Guo, . Z., Li, . X., Ji, . Y., Li, . T., Li, . M., Wang, . C., Chen, . J., Luo, . Y. & Liu, . X. (2024) Effect of the COVID-19 epidemic on drug resistance of Staphylococcus aureus infection. Journal of Microbiology and Infectious Diseases, 14 (1), 13-20 . doi:10.5455/JMID.2024.v14.i1.3 |