| Research Article | ||
J Microbiol Infect Dis. 2024; 14(3): 131-140 J. Microbiol. Infect. Dis., (2024), Vol. 14(3): 131–140 Review Article Fowlpox vaccine in chicken’s skin: Persistence and the local immune response at the site of inoculationIbrahim Eldaghayes1,2*, Lisa Rothwell2,3, Michael Skinner2,4 and Pete Kaiser 2,3,†1Department of Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 2Institute for Animal Health, Compton, UK 3The Roslin Institute and R(D)SVS, University of Edinburgh, Edinburgh, UK 4Section of Virology, Department of Medicine, Imperial College London, London, UK †Deceased *Corresponding Author: Ibrahim Eldaghayes. Department of Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya. Email: ibrahim.eldaghayes [at] vetmed.edu.ly Submitted: 11/06/2024 Accepted: 15/09/2024 Published: 30/09/2024 © 2024 Journal of Microbiology and Infectious Diseases
ABSTRACTBackground: Fowlpox virus (FPV) has been used as a vector for many years. To date, surprisingly, very little data exist on the persistence of fowlpox vaccines in chicken tissues, or what kind of immune cells respond to vaccination at the site of inoculation. Although both humoral and cellular-mediated immunity plays a part in overall immunity against FPV, little is known regarding the cell-mediated immune responses to FPV infection. Aim: The main aim of this paper was to measure the persistence of the fowlpox vaccine in skin tissues following vaccination. Methods: One-day-old chicks were vaccinated with FPV FP9. Skin samples were collected from the site of inoculation for several days following vaccination to assess the presence of FPV, as well as other immune cells including macrophages, CD4+ T cells, CD8+ T cells, and B cells. Results: Results showed that FPV does not persist for long, and is cleared by 6 days post-vaccination (dpv). The response of immune cells (macrophages, B cells, CD4+, and CD8+ T cells) that infiltrated the site of vaccination was assessed. Conclusion: The results in this paper reflected the response at the site of inoculation following a single vaccination; therefore, further studies regarding viral persistence and infiltration of immune cells at the site of inoculation after booster vaccination should be done. Keywords: Fowlpox virus, FP9, Immune cells, Vaccination, Persistence. IntroductionWhile attenuated fowlpox virus (FPV) strains are widely used for vaccination of chickens and turkeys for prevention of fowlpox infection, recombinant FPVs expressing various foreign genes have been evaluated for their ability to offer protection against various diseases in poultry as well as in mammals (Criado et al., 2019; Chen et al., 2020; Zhao et al., 2020; Portal et al., 2020; Hein et al., 2021; Zanotto et al., 2021; Eldaghayes et al., 2023). FPV has been used as a vector for quite a long time. To date, surprisingly, very little data exist on the persistence of fowlpox vaccines in chicken tissues, or what kind of immune cells respond to vaccination at the site of inoculation. Although both humoral and cellular-mediated immunity (CMI) play a part in overall immunity against FPV, little is known regarding the cell-mediated immune responses to FPV infection. It was shown that FPV infection causes a lymphoproliferative response in chickens and that the majority of proliferating T cells, largely CD8+, were virus antigen-specific (Singh and Tripathy, 2003). The main aim of this paper was to measure the persistence of the fowlpox vaccine in the skin tissues of chickens following vaccination. The skin has an important role in innate and adaptive immunity. It is well known that most microorganisms that come into contact with the skin barrier do not penetrate it. In addition, the blood and lymphatic networks act as highways on which immune cells travel to get to and from their sites of action. The skin houses many immunological cells. Since the primary function of the skin is to provide an effective barrier against the outside world, it is likely that a rapid and efficient host defense system exists and can be triggered once the barrier has been broken. Materials and MethodsChickensRhode Island Red chicks were obtained from an unvaccinated flock maintained in isolation accommodation at the Institute for Animal Health, Compton, UK. The experiments met with local ethical guidelines as well as those of the UK Home Office. VirusesFPV FP9 (Bayliss et al., 1991) was obtained from laboratory stocks. FP9 was grown on chicken embryo fibroblast (CEF) cells in the presence of 1X 199 medium (Sigma). Experimental designForty two chicks at 1 week of age were divided into three groups, 14 birds in each group as follows: Group 1 was inoculated with 50 µl phosphate-buffered saline (PBSa) per bird (negative control). Group 2 was inoculated with 250 µg/ml phytohaemagglutinin (PHA) per bird (positive control). Group 3 was inoculated with FP9. The vaccination dose of FP9 at 1 week of age was 107 pfu in a 50 ml volume. The inoculum was placed on both wing webs of each bird and the skin punctured 30 times over an area of 2 mm2 with a 21-gauge hypodermic needle. Two birds from each group were killed each time point by cervical dislocation and skin samples from the site of inoculation were taken. The first sample was taken 2 days post-inoculation and sampling was then carried out every 2 days till 14 days post-inoculation. Two skin samples from each bird (both wing webs) were taken one for real-time quantitative PCR to measure the viral load, and the other for immunohistochemical staining (for FPV, macrophages, CD4+ T cells, CD8+ T cells, and B cells), to estimate the relative number of positively-staining cells at the site of inoculation using Image-Pro® Plus software, version 4.0. Two skin sections (from two birds) and three fields of view per skin section were used at each time point for image analysis. This is a semi-quantity measurement, as it gives the percentage of the whole microscopic field (per area) that stained positive. DNA extractionUp to 25 mg of skin was cut into small pieces, and placed in a 1.5 ml micro-centrifuge tube. DNeasy tissue kit from QIAGEN was used for rapid isolation of total DNA following the manufacturer’s instructions. Frozen sections for immunohistochemical stainingEach skin sample was put on a 2.5 cm2 cork tile and covered with Tissue-Tek® O.C.TTM compound. The samples were then snap-frozen in a dry-ice/iso-pentane bath and transferred to liquid nitrogen. Frozen blocks were then removed from the liquid nitrogen, wrapped in aluminum foil, and stored at −70oC. Sections (6–8 mm) were then cut from these blocks for immunohistochemistry staining using a cryostat, picked up onto glass slides, then fixed in acetone for 10 minutes, and air-dried. Staining was then carried out using a Vectastain® ABC αmouse IgG HPR staining kit (Vector Laboratories, Burlingame, CA, USA), following the manufacturer’s instructions. The monoclonal antibodies used were DF6 (Boulanger et al., 2002) for FPV, KUL01 (Mast et al., 1998) for macrophages, AV14 (Tregaskes et al., 1995) for CD8, AV29 [Fred Davison, IAH] for CD4, and AV20 (Rothwell et al., 1996) for B cells. Table 1. Primers and probes for real-time quantitative PCR.
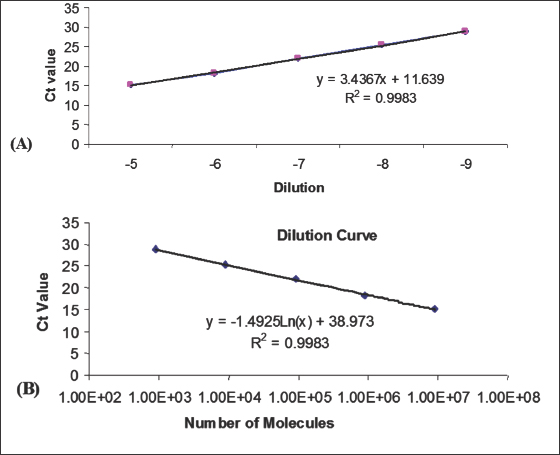
Real-time quantitative PCRThe fluorescently labeled probes were labeled with the reporter dye 5-carboxyfluoroscein (FAM) at the 5′ end and the quencher N,N,N’,N’–tetramethyl-6-carboxyrhodamine (TAMRA) at the 3′ end. Specific primers were designed to closely flank the probe. Primers and probes sequences are given in Table 1. Amplification and detection of specific products were undertaken using the ABI PRISM™ 7700 Sequence Detection System. Quantification was based on increased fluorescence detected by the ABI PRISM™ 7700 Sequence Detection System (PE Applied Biosystems) due to hydrolysis of the target-specific probes by the 5′ nuclease activity of the rTth DNA polymerase during PCR amplification. Results were expressed in terms of the threshold cycle value (Ct), the cycle at which the change in the reporter dye (ΔRn) passes a significance threshold. Construction of standard curves for quantitative PCR assaysStandard curves for the DNA-specific reactions were generated from real-time PCR reactions on serial log10 dilutions of DNA standards (usually plasmid DNA containing the same DNA template) and were included in every experiment. Dilutions of 10−1 to 10−9 were made in DEPC-H2O. Regression analysis of the mean values for the log10 diluted DNA was used to generate a standard curve (Fig. 1). Ethical approvalThis study was carried out in strict accordance with the Animals (Scientific Procedures) Act 1986, an Act of Parliament of the United Kingdom, following approval by the Ethical Review Committee of the ethics committee of Institute for Animal Health, Compton, Berkshire, UK, and the United Kingdom Government Home Office under the project license 70/7781.
Fig. 1. Real-time quantitative PCR. (A): Standard curve. (B): Number of molecules per Ct value.
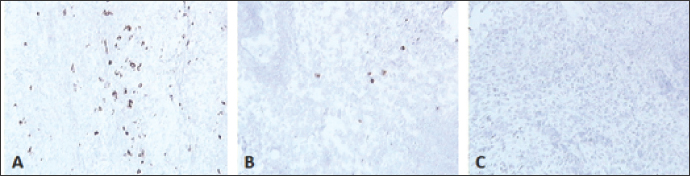
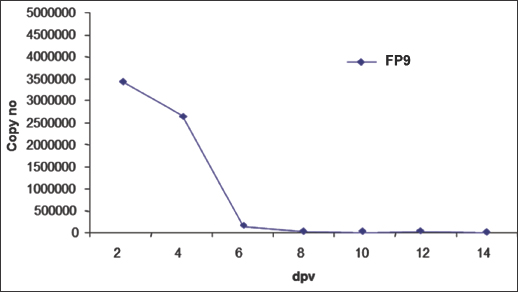
Fig. 2. Skin sections of birds vaccinated with FP9 and stained with monoclonal antibody DF6 against FPV. (A) 2 dpv, (B) 4 dpv, and (C) 6 dpv. Positive stained cells (cells infected with the FPV) appeared brown (100x magnification). ResultsFP9 persistence in tissue following vaccinationUsing immunohistochemical stainingSkin sections from the site of vaccination were stained using a specific monoclonal antibody (DF6) against FPV (Boulanger et al., 2002). FP9 was detected at two dpv and to a lower degree at 4 dpv. No virus was detected from 6 dpv onwards (Fig. 2). Using real-time quantitative PCRDNA was extracted from the skin at the site of vaccination. Real-time quantitative PCR was carried out to detect the virus in the skin tissue. A very high number of viral copies was detected at 2 dpv and 4 dpv, whereas very low viral titers were detected from 6 dpv onwards (Fig. 3). Influence of immune cells at the site of vaccinationMacrophagesSkin sections were stained using the monoclonal antibody KUL01 (Mast et al., 1998) (Fig. 4). The pattern of macrophage infiltration at the site of vaccination in the skin was similar to that of the virus, as high numbers were detected at 2 dpv and 4 dpv, and these dropped from 6 dpv onwards (Fig. 5).
Fig. 3. Copy number of the viral vaccine (FP9) in the skin following vaccination, using real-time quantitative PCR.
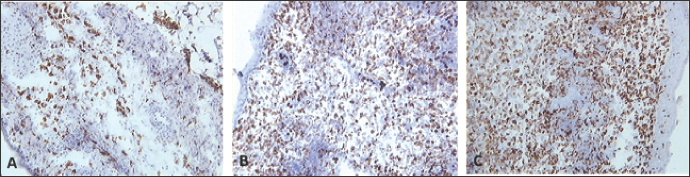
Fig. 4. Skin sections of birds stained with monoclonal antibody KUL01 against macrophages. Two days post inoculation with (A) PBSa, (B) PHA, and (C) FP9. Positive staining for macrophages appeared brown (100x magnification).
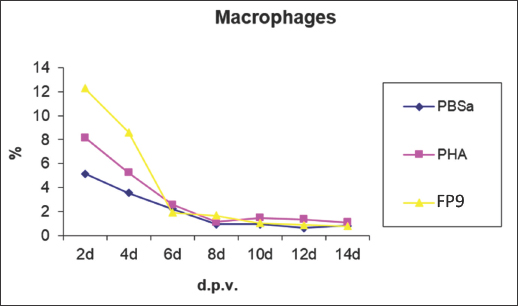
Fig. 5. Kinetics of macrophage numbers in skin sections of birds vaccinated with FP9. % represents the percentage of the field of view staining positive with an anti-macrophage monoclonal antibody (KUL01), PBSa represented the negative control whereas PHA represented the positive control.
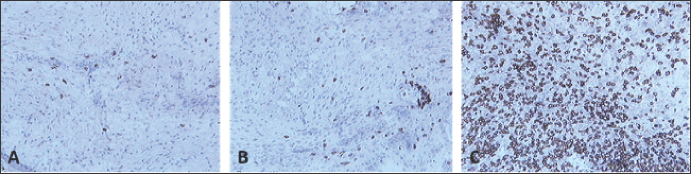
Fig. 6. Skin sections of birds stained with the monoclonal antibody AV296 days post inoculation with (A) PBSa, (B) PHA, and (C) FP9. Positive staining for CD4+ cells appeared brown (100x magnification).
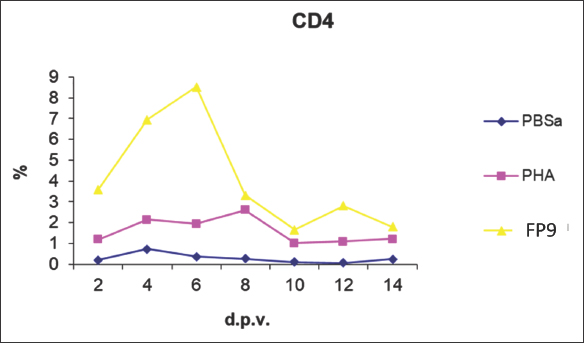
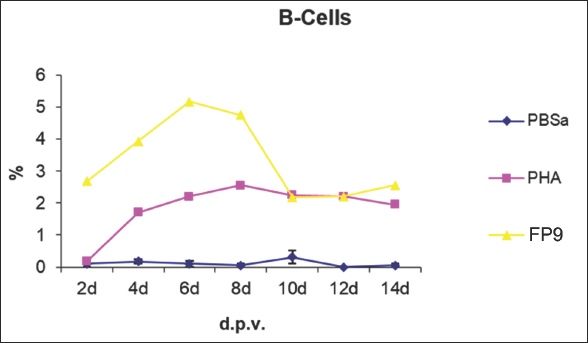
Fig. 7. Kinetics of CD4+ T cell numbers in skin sections of birds vaccinated with FP9. % represents the percentage of the field of view staining positive with an anti-CD4+ T cell monoclonal antibody (AV29), PBSa represented the negative control whereas PHA represented the positive control. CD4+ T cellsSkin sections were stained with the monoclonal antibody AV29, which recognizes CD4+ T cells (Davison, IAH) (Fig. 6). Numbers of CD4+ cells increased slightly from 2 dpv compared to birds inoculated with PBSa and PHA. CD4+ T cells gradually increased to reach a peak at 6 dpv at the site of vaccination in the skin, followed by a gradual decrease from 8 dpv onwards (Fig. 7). CD8+ T cellsSkin sections were stained with the monoclonal antibody AV14, which recognizes CD8+ T cells (Tregaskes et al., 1995) (Fig. 8). CD8+ cells increased rapidly and sharply starting from 4 dpv, and reached a peak at 6 dpv at the site of vaccination in the skin, and then dropped rapidly from 8 dpv onwards (Fig. 9). B cellsSkin sections were stained with the monoclonal antibody AV20 which recognizes Bu-1 on B cells (Rothwell et al., 1996) (Fig. 10). B cells increased in number from 2 dpv to reach a peak at 6–8 dpv at the site of vaccination in the skin, and then dropped in number from 10 dpv onwards (Fig. 11). At 2 and 4 dpv, B cells in skin sections were diffused. The formation of germinal centers (GC)-like structures started appearing at 6 dpv (Fig. 10). At 8 dpv onwards, almost all B cells were aggregated in these GC-like structures. DiscussionPrevious studies indicate that an intradermal inoculation of FPV (both challenge and vaccine strains) into the comb of birds resulted in primary lesions that were restricted to the site of inoculation (Minbay and Kreier, 1973). Local multiplication resulted in the development of lesions at the site of virus entry. Typically, primary lesions appeared by the Fourth day and disappeared by the third week. No secondary lesions developed in any of the birds. No virus was detected in the blood, and the internal organs were free of virus during the infection (Minbay and Kreier, 1973). The challenge FPV was detected on the second day from the lesion samples collected from the comb (site of inoculation). Thereafter, the virus titer increased rapidly to a maximum on the ninth day, and then fell from the 12th day onwards. No virus was detected at 27 dpi. Virus persistence was shorter and virus concentration was lower with the vaccine strain than the challenge strain (Minbay and Kreier, 1973). Another study (Singh et al., 1987) showed that in chickens infected with fowlpox intradermally, the virus was detected in the lungs at 4 dpi which was followed by viremia, at 5 dpi.
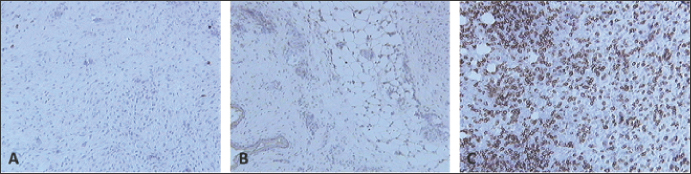
Fig. 8. Skin sections of birds stained with the monoclonal antibody AV14 6 days post inoculation with (A) PBSa, (B) PHA, and (C) FP9. Positive staining for CD8+ cells appeared brown (100x magnification).
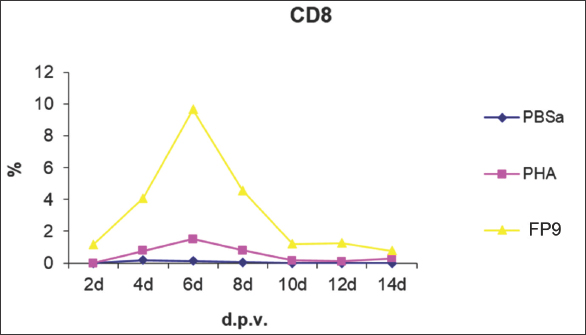
Fig. 9. Kinetics of CD8+ T cell numbers in skin sections of birds vaccinated with FP9. % represents the percentage of the field of view staining positive with an anti-CD8+ T cell monoclonal antibody (AV14), PBSa represented the negative control whereas PHA represented the positive control.
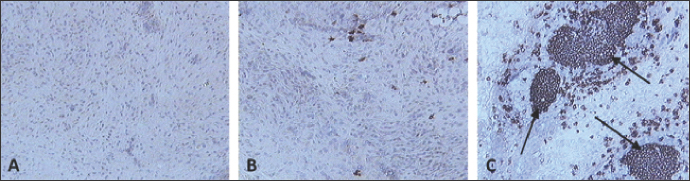
Fig. 10. Skin sections of birds stained with the monoclonal antibody AV20 6 days post inoculation with (A) PBSa, (B) PHA, and (C) FP9. Positive staining for B cells appeared brown. Arrows indicate formation of GC-like structures (100x magnification).
Fig. 11. Kinetics of B cell numbers in skin sections of birds vaccinated FP9. % represents the percentage of the field of view staining positive with an anti-B cell monoclonal antibody (AV20). PBSa represented the negative control whereas PHA represented the positive control. FPV strain FP9 was used in the present study. Its genome has been completely sequenced (Laidlaw and Skinner, 2004), allowing identification of all of the differences (including deletions totaling 22 kb) between it and the USDA standard challenge virus, which was described in the original sequence publication as “pathogenic” (Afonso et al., 2000). FP9 (FPV plaque 9) was derived by plaque purification of a virus that had been passaged some 438 times in CEF culture, the source isolates being HP-1 Munich (Mayr, 1960). Any residual virulence (even for day-old chicks) had been lost by passage 350 (Mayr and Malicki, 1966). FP9 is highly attenuated and possibly introduces genetic deletions/modifications that enhance the immune response elicited by this vector. A comparison of the FP9 genome sequence with the published sequence of a pathogenic FPV reference strain (Afonso et al., 2000) revealed several inserted as well as deleted sequences (Laidlaw and Skinner, 2004). Such deletions/insertions may account for the enhanced capacity of FP9 to elicit T cell responses. This may explain the short persistence of FP9 used in the present study compared to the long persistence for the challenge FPV used by Minbay and Kreier (Minbay and Kreier, 1973). In terms of histopathology, hyperplasia of the epithelium, enlargement of cells and the presence of eosinophilic cytoplasmic inclusion bodies (Bollinger bodies) are common for FPV infection (Giotis and Skinner, 2019). It was interesting to determine which cell types were involved at the site of FP9 inoculation. By virtue of their location at various sites in the body, macrophages are one of the first cells to encounter viruses (Lazarov et al., 2023). The immunological function of the epidermis is principally linked to the presence in this tissue of a distinct subpopulation of Langerhans cells (LC) or macrophages (Neagu et al., 2022). In man, LC constitute 2%–4% of the epidermal cell population, and within the epidermis, they are the only cells that express MHC class II antigens constitutively (Hasegawa et al., 2004). LC plays a key role in the initiation of T cell responses to cutaneous antigens by picking up antigens and migrating to the draining lymph node (in mammals), where they trigger specific T cell activation (Neagu et al., 2022). In our experiment, the skin was inoculated with PBSa as a negative control and PHA as a positive control. In vitro, PHA stimulates T cell proliferation and differentiation, while in vivo the PHA-skin response is an inflammatory reaction, concerning complex interactions of cells—not only lymphocytes but also macrophages and basophils (Hasegawa et al., 2004). In mammals, when an antigen has passed the epithelial barrier of the skin or mucosal surfaces it has to be processed and presented by accessory cells to lymphocytes. These reactions take place in lymphoid organs, such as the regional lymph nodes, Peyer’s patches, and tonsils, but also in the spleen if the antigen enters the blood directly. The respective lymphocyte clone expands by proliferating, and primed lymphocytes of the B and T cell series emigrate from the lymphoid organs (Chambers and Vukmanovic-Stejic, 2020). As there are no lymph nodes in chickens, antigen presentation to lymphocytes may occur within the skin tissue (Lu et al., 2023). From Figures 2 and 3 of the present study, the virus was detected in skin tissue after vaccination at a very high concentration at 2 dpv, to a lower degree at 4 dpv and was almost cleared from 6 dpv. The pattern of macrophage infiltration at the site of inoculation in the skin tissue was similar to that of the virus, in that high numbers were observed at 2 dpv and 4 dpv, and these dropped from 6 dpv onwards (Figs. 4 and 5). The concentration of macrophages in the skin at the site of inoculation was higher than that for the birds which were inoculated with PBSa (negative control) and PHA (inflammatory agent), suggesting that the virus might have been taken up by macrophages. In vitro, PHA stimulates T cell proliferation and differentiation while in vivo, the PHA-skin response is an inflammatory reaction, concerning complex interactions of cells—not only lymphocytes but also macrophages and basophils (Restifo et al., 1994). The use of specific monoclonal antibodies coupled with image analysis (Image pro-plus software), made it easy to assess changes in the quantity of target cells at the site of vaccination. Results of the experiments in the present study suggest that both CD4+ and CD8+ T cells participated in the response. Intracellular organisms (usually viruses) synthesize proteins inside the infected cells, and these antigens therefore gain ready access to the MHC class I pathway; as a result, they often induce strong MHC class I restricted CD8+ T cell responses (these cells are usually cytotoxic, although secrete a variety of cytokines in response to antigen) (Reina-Campos et al., 2021). From Figure 9 of the present study, a rapid increase of CD8+ T cells in skin sections from 4 dpv was observed, which peaked at 6 dpv, at which the virus was almost cleared. Furthermore, antigens synthesized during intracellular infections are usually released from the infected cells into the extracellular matrix, and these soluble materials are taken up by specialized antigen-presenting cells (APCs). Inside these APCs, antigens enter the MHC class II pathway, permitting them to induce MHC class II-restricted CD4+ T cells, which usually provide “help” to B cells. The soluble antigens also encounter B lymphocytes and induce antibody responses (in concert with CD4+ T cell “help”). Therefore, most intracellular organisms are expected to induce strong CD4+, CD8+, and B cell responses (Figs. 7, 9, and 11, respectively). Recombinant FPV expressing tumor (Wang et al., 1995; Irvine et al., 1997; Grosenbach et al., 2001) and HIV antigens (Robinson et al., 2000; Vazquez et al., 2002; Zhu et al., 2017) elicit CD8+ T cell responses in rodents. In addition, studies with non-human primates have shown that recombinant FPV encoding HIV antigens can boost the immune response primed by a DNA vaccine, leading to enhanced cytotoxic T cell responses and protection against viral challenge (Robinson et al., 2000). Recombinant FPV vectors can be used to elicit CD8+ T cell responses against Plasmodium berghei Malaria by prime-boost immunization regimens using a novel attenuated FPV (Anderson et al., 2004). Interestingly, FP9 was more effective in eliciting a response against the P. berghei circumsporozoite protein than the commercially available FPV vaccine strain (Anderson et al., 2004). B cells were not present in normal human skin (Bos et al., 1987). After vaccination, there was an aggregation of B cells at the site of vaccination. After antigen challenge, GC is formed within the secondary lymphoid organs (lymph nodes, Peyer’s patches, spleen, or tonsils), and from the present experiment, GC-like structures were formed in the skin tissue too (Fig. 10). At 2 and 4 dpv, B cells in skin sections were diffused. However, the formation of GC-like structures started at 6 dpv (Fig. 10). At 8 dpv onwards, almost all B cells were aggregated in these GC-like structures. GC are the sites from where B cells grow and differentiate to immunoglobulin-producing plasma cells, generate high-affinity antigen-specific B-cell receptors by affinity maturation, and differentiate into memory cells. However, after FP9 vaccination, detectable antibodies were produced against FPV (Bayliss et al., 1991; Shaw and Davison, 2000). For successful vaccination, “pock” lesions should appear at the site of inoculation within the first week after vaccination. Immunity will normally develop at 10–14 dpv (Bayliss et al., 1991). It was suggested that CMI plays a more important role in the recovery from FPV infection than humoral immunity (Morita, 1973). On the other hand, humoral immunity was not excluded since mortality was significantly higher in bursectomised-thymectomised chickens than in thymectomised chickens following FPV infection (Morita, 1973). The regulation of the entry of activated T and B lymphocytes into skin tissue is not well understood. There is also evidence that keratinocytes participate in immune responses in the skin since these cells produce a variety of cytokines that can modulate T cell responses (Morizane et al., 2023). ConclusionIn conclusion, FPV has been used as a vector for a long period. Surprisingly little data currently exists on the persistence of fowlpox vaccines in chicken tissues at the site of inoculation, or on what kind of local immune response there is to the vaccination at the site of vaccine inoculation. Results of the present study showed that FPV do not persist for long, and are cleared by 6 dpv. We recommend further studies regarding viral persistence and infiltration of immune cells at the site of inoculation after booster vaccination, as all the results in this paper reflected the response at the site of inoculation after a prime vaccination. AcknowledgmentsThe authors would like to thank Stephen Laidlaw, Fred Davison, Colin Butter, the people in the EAH, especially Don Hoopre, the Media people, especially Richard Oaks, and the Histology lab people for their help. Conflicts of interestThe authors declare no conflict of interest. FundingThis research was funded by the Libyan Government through the Libyan Embassy in London as a support for Ibrahim Eldaghayes’s PhD; Grant No. 01/2001. Authors’ contributionsConceptualization, I.E. and P.K.; methodology, I.E.; validation, I.E., L.R., M.S., and P.K.; investigation, I.E.; data curation, I.E., and L.R.; writing—original draft preparation, I.E.; writing—review and editing, I.E., L.R., M.S., and P.K.; supervision, P.K. All authors have read and agreed to the published version of the manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. Any additional data are available from the corresponding author upon reasonable request. ReferencesAfonso, C.L., Tulman, E.R., Lu, Z., Zsak, L., Kutish, G.F. and Rock, D.L. 2000. The genome of fowlpox virus. J. Virol. 74, 3815–3831. Anderson, R.J., Hannan, C.M., Gilbert, S.C., Laidlaw, S.M., Sheu, E.G., Korten, S., Sinden, R., Butcher, G.A., Skinner, M.A. and Hill, A.V. 2004. Enhanced CD8+ T cell immune responses and protection elicited against Plasmodium berghei malaria by prime boost immunization regimens using a novel attenuated fowlpox virus. J. Immunol. 172, 3094–3100. Bayliss, C.D., Peters, R.W., Cook, J.K., Reece, R.L., Howes, K., Binns, M.M. and Boursnell, M.E. 1991. A recombinant fowlpox virus that expressed the VP2 antigen of infectious bursal disease virus induces protection against mortality caused by the virus. Arch. Virol. 120, 193–205. Bos, J.D., Zonneveld, I., Das, P.K., Krieg, S.R., van der Loos, C.M. and Kapsenberg, M.L. 1987. The skin immune system (SIS): distribution and immunophenotype of lymphocyte subpopulations in normal human skin. J. Invest. Dermatol. 88, 569–573. Boulanger, D., Green, P., Jones, B., Henriquet, G., Hunt, L.G., Laidlaw, S.M., Monaghan, P. and Skinner, M.A. 2002. Identification and characterization of three immunodominant structural proteins of fowlpox virus. J. Virol. 76, 9844–9855. Chambers, E.S. and Vukmanovic-Stejic, M. 2020. Skin barrier immunity and ageing. Immunology 160, 116–125; doi: 10.1111/imm.13152. Chen, S., Xu, N, Ta, L., Li, S., Su, X., Xue, J., Du, Y., Qin T. and Peng, D. 2020. Recombinant fowlpox virus expressing gB gene from predominantly epidemic infectious Larygnotracheitis virus strain demonstrates better immune protection in SPF chickens. Vaccines (Basel) 8, 623; doi: 10.3390/vaccines8040623. Criado, M.F., Bertran, K., Lee, D.H., Killmaster, L., Stephens, C.B., Spackman, E., SaESilva, M., Atkins, E., Mebatsion, T., Widener, J., Pritchard, N., King, H. and Swayne, D.E. 2019. Efficacy of novel recombinant fowlpox vaccine against recent Mexican H7N3 highly pathogenic avian influenza virus. Vaccine 37, 2232–2243; doi: 10.1016/j.vaccine.2019.03.009. Eldaghayes, I., Rothwell, L., Skinner, M., Dayhum, A. and Kaiser, P. 2023. Efficacy of fowlpox virus vector vaccine expressing VP2 and chicken interleukin-18 in the protection against Infectious Bursal Disease Virus. Vaccines (Basel) 11, 1716; doi: 10.3390/vaccines11111716. Giotis, E.S. and Skinner, M.A. 2019. Spotlight on avian pathology: fowlpox virus. Avian Pathol. 48, 87–90; doi: 10.1080/03079457.2018.1554893. Grosenbach, D.W., Barrientos, J.C., Schlom, J. and Hodge, J.W. 2001. Synergy of vaccine strategies to amplify antigen-specific immune responses and antitumor effects. Cancer Res. 61, 4497–4505. Hasegawa, J., Kashima, Y., Matsuda, M., Kawano, M. and Wakimoto, T. 2004. Effects of in ovo exposure to PCBs (coplanar congener, kanechlor mixture, hydroxylated metabolite) on the developing cell-mediated immunity in chickens. Develop. Reprod. Toxi. 66, 3082–3086. Hein, R., Koopman, R., García, M., Armour, N., Dunn, J.R., Barbosa, T. and Martinez, A. 2021. Review of poultry recombinant vector vaccines. Avian Dis. 65, 438–452; doi: 10.1637/0005-2086-65.3.438. Irvine, K.R., Chamberlain, R.S., Shulman, E.P., Surman, D.R., Rosenberg, S.A. and Restifo, N.P. 1997. Enhancing efficacy of recombinant anticancer vaccines with prime/boost regimens that use two different vectors. J. Natl. Cancer Inst. 89, 1595–1601. Laidlaw, S.M. and Skinner, M.A. 2004. Comparison of the genome sequence of FP9, an attenuated, tissue culture-adapted European strain of Fowlpox virus, with those of those virulent American and European viruses. J. Gen. Virol. 85, 305–322. Lazarov, T., Juarez-Carreño, S., Cox, N. and Geissmann, F. 2023. Physiology and diseases of tissue-resident macrophages. Nature 618, 698–707; doi: 10.1038/s41586-023-06002-x. Lu, M., Lee, Y. and Lillehoj, H.S. 2023. Evolution of developmental and comparative immunology in poultry: the regulators and the regulated. Dev. Comp. Immunol. 138, 104525; doi: 10.1016/j.dci.2022.104525. Mast, J., Goddeeris, B.M., Peeters, K., Vandesande, F. and Berghman, L.R. 1998. Characterisation of chicken monocytes, macrophages and interdigitating cells by the monoclonal antibody KUL01. Vet. Immunol. Immunopathol. 61, 343–357. Mayr, A. 1960. The behavior of chicken, pigeon and canary pock viruses in the chick after intravenous inoculation. Arch. Hyg. Bacteriol. 179, 149–159. Mayr, A. and Malicki, K. 1966. Attenuation of virulent Fowlpox virus in tissue culture and characteristics of the attenuated virus. Zentralbl. Veterinarmed. B. 13, 1–13. Minbay, A. and Kreier, J.P. 1973. An experimental study of the pathogenesis of fowlpox infection in chickens. Avian Dis. 17, 532–539. Morita, C. 1973. Role of humoral and cell-mediated immunity on the recovery of chickens from fowlpox virus infection. J. Immunol. 111, 1495–1501. Morizane, S., Mukai, T., Sunagawa, K., Tachibana, K., Kawakami, Y. and Ouchida, M. 2023. “Input/output cytokines” in epidermal keratinocytes and the involvement in inflammatory skin diseases. Front Immunol. 14, 1239598; doi: 10.3389/fimmu.2023.1239598. Neagu, M., Constantin, C., Jugulete, G., Cauni, V., Dubrac, S., Szöllősi, A.G. and Zurac, S. 2022. Langerhans cells-revising their role in skin pathologies. J. Pers. Med. 12, 2072; doi: 10.3390/jpm12122072. Portal, D.E., Weiss, R.E., Wojtowicz, M., Mansour, A., Monken, C., Mehnert, J.M., Aisner, J.A., Kane, M., Nishioka, J., Aisner, S., Peters, S., Stein, M.N., Kim, I.Y., Mayer, T.M., Shih, W., Gulley, J., Streicher, H., Singer, E.A. and Lattime, E.C. 2020. Phase I neoadjuvant study of intravesical recombinant fowlpox-GM-CSF (rF-GM-CSF) or fowlpox-TRICOM (rF-TRICOM) in patients with bladder carcinoma. Cancer Gene Ther. 27, 438–447; doi: 10.1038/s41417-019-0112-z. Reina-Campos, M., Scharping, N.E. and Goldrath, A.W. 2021. CD8+ T cell metabolism in infection and cancer. Nat. Rev. Immunol. 21, 718–738; doi: 10.1038/s41577-021-00537-8. Restifo, N.P., Minev, B.R., Taggarse, A.S., McFarland, B.J., Wang, M. and Irvine, K.R. 1994. Enhancing the recognition of tumour associated antigens. Folia. Biol. (Praha) 40, 74–88. Robinson, H.L., Montefiori, D.C., Johnson, R.P., Kalish, M.L., Lydy, S.L. and McClure, H.M. 2000. DNA priming and recombinant pox virus boosters for an AIDS vaccine. Dev. Biol. (Basel) 104, 93–100. Rothwell, C.J., Vervelde, L. and Davison, T.F. 1996. Identification of chicken Bu-1 alloantigens using the monoclonal antibody AV20. Vet. Immunol. Immunopathol. 55, 225–234. Shaw, I. and Davison, T.F. 2000. Protection from IBDV-induced bursal damage by a recombinant fowlpox vaccine, fpIBD1, is dependent on the titre of challenge virus and chicken genotype. Vaccine 18, 3230–3241. Singh, G.K., Singh, N.P. and Garg, S.K. 1987. Studies on pathogenesis of fowl pox: Virological study. Acta Virol. 31, 417–423. Singh, P. and Tripathy, D.N. 2003. Fowlpox virus infection causes a lymphoproliferative response in chickens. Viral Immunol. 16, 223–227. Tregaskes, C.A., Kong, F.K., Paramithiotis, E., Chen, C.L., Ratcliffe, M.J., Davison, T.F. and Young, J.R. 1995. Identification and analysis of the expression of CD8 alpha beta and CD8 alpha alpha isoforms in chickens reveals a major TCR-gamma delta CD8 alpha beta subset of intestinal intraepithelial lymphocytes. J. Immunol. 154, 4485–4494. Vazquez, B.D., Green, P., Laidlaw, S.M., Skinner, M.A., Borrow, P. and Duarte, C.A. 2002. Induction of a strong HIV-specific CD8+ T cell response in mice using a fowlpox virus vector expressing an HIV-1 multi-CTL-epitope polypeptide. Viral Immunol. 15, 337–356. Wang, M., Bronte, V., Chen, P.W., Gritz, L., Panicali, D., Rosenberg, S.A. and Restifo, N.P. 1995. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J. Immunol. 154, 4685–4692. Zanotto, C., Paolini, F., Radaelli, A. and De Giuli Morghen, C. 2021. Construction of a recombinant avipoxvirus expressing the env gene of Zika virus as a novel putative preventive vaccine. Virol. J. 18, 50; doi: 10.1186/s12985-021-01519-x. Zhao, Y., Han, Z., Zhang, X., Zhang, X., Sun, J., Ma, D. and Liu, S. 2020. Construction and immune protection evaluation of recombinant virus expressing Newcastle disease virus F protein by the largest intergenic region of fowlpox virus NX10. Virus Genes 56, 734–748; doi: 10.1007/s11262-020-01799-5. Zhu, Y., Guo, Y., Du, S., Liu, C., Wang, M., Ren, D., Zhao, F., Zhang, Y., Sun, W., Li, Y., Cao, T., Jiang, Y., Xing, B., Bai, B., Li, C. and Jin, N. 2017. Construction, selection and immunogenicity of recombinant fowlpox candidate vaccine co-expressing HIV-1 gag and gp145. Indian J Microbiol. 57, 162–170; doi: 10.1007/s12088-017-0639-3. | ||
| How to Cite this Article |
| Pubmed Style Eldaghayes I, Rothwell L, Skinner M, Kaiser P. Fowlpox virus in chicken’s skin: Persistence and the local immune response at the site of inoculation. J Microbiol Infect Dis. 2024; 14(3): 131-140. doi:10.5455/JMID.2024.v14.i3.6 Web Style Eldaghayes I, Rothwell L, Skinner M, Kaiser P. Fowlpox virus in chicken’s skin: Persistence and the local immune response at the site of inoculation. https://www.jmidonline.org/?mno=220668 [Access: January 25, 2026]. doi:10.5455/JMID.2024.v14.i3.6 AMA (American Medical Association) Style Eldaghayes I, Rothwell L, Skinner M, Kaiser P. Fowlpox virus in chicken’s skin: Persistence and the local immune response at the site of inoculation. J Microbiol Infect Dis. 2024; 14(3): 131-140. doi:10.5455/JMID.2024.v14.i3.6 Vancouver/ICMJE Style Eldaghayes I, Rothwell L, Skinner M, Kaiser P. Fowlpox virus in chicken’s skin: Persistence and the local immune response at the site of inoculation. J Microbiol Infect Dis. (2024), [cited January 25, 2026]; 14(3): 131-140. doi:10.5455/JMID.2024.v14.i3.6 Harvard Style Eldaghayes, I., Rothwell, . L., Skinner, . M. & Kaiser, . P. (2024) Fowlpox virus in chicken’s skin: Persistence and the local immune response at the site of inoculation. J Microbiol Infect Dis, 14 (3), 131-140. doi:10.5455/JMID.2024.v14.i3.6 Turabian Style Eldaghayes, Ibrahim, Lisa Rothwell, Michael Skinner, and Pete Kaiser. 2024. Fowlpox virus in chicken’s skin: Persistence and the local immune response at the site of inoculation. Journal of Microbiology and Infectious Diseases, 14 (3), 131-140. doi:10.5455/JMID.2024.v14.i3.6 Chicago Style Eldaghayes, Ibrahim, Lisa Rothwell, Michael Skinner, and Pete Kaiser. "Fowlpox virus in chicken’s skin: Persistence and the local immune response at the site of inoculation." Journal of Microbiology and Infectious Diseases 14 (2024), 131-140. doi:10.5455/JMID.2024.v14.i3.6 MLA (The Modern Language Association) Style Eldaghayes, Ibrahim, Lisa Rothwell, Michael Skinner, and Pete Kaiser. "Fowlpox virus in chicken’s skin: Persistence and the local immune response at the site of inoculation." Journal of Microbiology and Infectious Diseases 14.3 (2024), 131-140. Print. doi:10.5455/JMID.2024.v14.i3.6 APA (American Psychological Association) Style Eldaghayes, I., Rothwell, . L., Skinner, . M. & Kaiser, . P. (2024) Fowlpox virus in chicken’s skin: Persistence and the local immune response at the site of inoculation. Journal of Microbiology and Infectious Diseases, 14 (3), 131-140. doi:10.5455/JMID.2024.v14.i3.6 |