| Research Article | ||
J Microbiol Infect Dis. 2023; 13(3): 128-138 J. Microbiol. Infect. Dis., (2023), Vol. 13(3): 128–138 Original Research Assessment of thyroid function and biochemical markers among COVID-19 Libyan patientsMouna Y. Ben Omar1,2, Abdunnabi A. Rayes3, Nagat S. Alkmishi4, Firuz A. Elaswad4, Hanadi A. Ben Younes5, Munay S. BinGhisheer5, Ibtehal A. Fadli5, Abdunaser S. Dayhum6 and Ibrahim M. Eldaghayes7*1Laboratories Department, Faculty of Medical Technology, University of Tripoli, Tripoli, Libya 2Zoology Department, Faculty of Sciences, University of Tripoli, Tripoli, Libya 3Department of Internal Medicine, Faculty of Medicine, University of Tripoli, Tripoli, Libya 4Medical Documentation and Statistics Department, Reference Medical Laboratory, Tripoli, Libya 5Zaweit Al-Dahmani Polyclinic, Ministry of Health, Tripoli, Libya 6Department of Preventive Medicine, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 7Department of Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya *Corresponding Author: Ibrahim M. Eldaghayes. Department of Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya. Email: ibrahim.eldaghayes [at] vetmed.edu.ly Submitted: 03/08/2023 Accepted: 16/09/2023 Published: 30/09/2023 © 2023 Journal of Microbiology and Infectious Diseases
AbstractBackground: There is a scarcity of data on thyroid function abnormality in coronavirus disease 2019 (COVID-19) outpatients in the world literature since previous studies were done on admitted patients. Aim: The objective of this study was to assess thyroid function tests in Libyan outpatients with COVID-19 as well as the possible association between them and some routine hematological, inflammatory, and biochemical markers. Methods: Laboratory results were retrospectively reviewed for a total number of 246 patients, where 214 patients with laboratory-confirmed COVID-19 and 32 non-COVID-19 patients were included in the study as a control group. The majority of the patients were females 179 (72.8%) and age range between 18 and 88 years old. They were registered in the outpatient department of COVID-19 at Zaweit-Dahmany Polyclinic in Tripoli, Libya between May and October 2021. Serum levels of thyroid-stimulating hormone, thyroid hormones (THs) [triiodothyronine (T3), thyroxine (T4), and free fractions], complete blood count, C-reactive protein (CRP), lactate dehydrogenase (LDH), liver function test, and renal function test were measured. Results: Abnormal thyroid function was seen in 17.8% of 214 patients with COVID-19. Twelve patients had isolated low total or free triiodothyronine (FT3), suggestive of nonthyroidal illness syndrome, ten patients had hypothyroidism that was subclinical in six patients and overt in the remaining four patients. Three patients had hyperthyroidism. Thirteen patients had different isolated THs abnormalities. Low FT3 was associated with older age (p=0.035), and it has a weak negative correlation with CRP (−0.335) and LDH (−0.245) (p=0.001). The thyroid dysfunction (TD) group presented a statistically significant reduction in lymphocytes (p=0.000), increased neutrophil (p=0.000), increased CRP (p=0.000), increased urea (p=0.014), increased alkaline phosphatase (p=0.007), a slight reduction in hematocrit (p=0.010), low mean corpuscular volume (p=0.019), and low mean corpuscular hemoglobin (p=0.019) but no significant difference in hemoglobin, red blood cells, white blood cells, and platelet count when compared to euthyroid control. Conclusion: Clinicians should be vigilant about the possible presence of thyroid function abnormalities among COVID-19 patients, especially elderly patients, and those with increased inflammatory markers. Keywords: COVID-19, Thyroid dysfunction, Biochemical markers, Inflammatory markers, Hematological markers. IntroductionCoronavirus disease-2019 (COVID-19) was first detected in Wuhan, China, by the end of 2019. Since then, the disease spread rapidly all over China and from there to the rest of the world. The causative agent of the disease was named a severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) (An et al., 2020), and it was announced as a global pandemic by WHO on the 11th of March 2020 (Aloisio et al., 2020). On the 24th of March 2020, the first patient with COVID-19 was diagnosed in Libya, and after this, the number of patients increased continuously (Elhadi et al., 2020). COVID-19 is related to multi-organ involvement consisting of respiratory, immune, hematological, digestive, hepatic, and renal structures due to the widespread expression of Angiotensin-converting enzyme 2, (ACE-2) receptor and, transmembrane protease serine 2, (TMPRSS2) that is the practical access sites for SARS-CoV-2 (Wang et al., 2021). The virus additionally influences the endocrine system, for example, ACE2 and TMPRSS2 are more highly expressed in the thyroid gland than inside the lungs. It additionally has feasible mechanisms that might affect the thyroid gland and its hypothalamic-pituitary axis through direct virus contamination or oblique immune-inflammatory response (Scappaticcio et al., 2020). The activation of the immune inflammatory response-mediated glandular harm through the formation of antibodies, as a result of humoral immune response, or cell-mediated damage to the thyroid gland, as a result of cellular immune response (Khoo et al., 2021). Therefore, thyroid characteristic abnormalities may be a complication of COVID-19. Numerous previous articles have defined the onset of thyroid disorder (TD) in normal thyroid among COVID-19 patients from subclinical to overt TD either hypo or hyperthyroidism in addition to nonthyroidal illness syndrome (NTIS) (Daraei et al., 2020; Lania et al., 2020; Giovanella et al., 2021). Few studies have mentioned an association between TD and inflammatory markers. Lui et al. (2021b) determined that a higher C-reactive protein (CRP) level, was independently linked to low free triiodothyronine (FT3). Wang et al. (2021) confirmed that the TD group had lower ranges of lymphocytes and higher ranges of leukocytes, neutrophils, CRP, and procalcitonin. Ilera et al. (2021) reported that in hospitalized patients with COVID-19, thyroid hormones (THs) correlated with inflammatory parameters and worse clinical effects mainly, a low triiodothyronine/thyroxine (T3/T4) ratio, was correlated with severity and poor prognosis. Patients who were examined showed low T3 but high free thyroxine (FT4), higher ferritin, lower albumin, and more severe disease. Moreover, it is wise on the part of healthcare providers to adopt a cautious approach when treating COVID-19 patients with altered thyroid levels as they can predict disease severity and correlate with biomarkers (Asghar et al., 2021). To the best of our information, this is the first study on this subject within the Libyan population with COVID-19 patients and may be the first examination achieved on outpatients in the literature since previous studies had been all done on admitted patients. This study aimed to assess the effect of COVID-19 on TH levels and to correlate them with some hematological and biochemical markers in Libyan patients stricken by COVID-19. Materials and MethodsPatientsAll patients with laboratory-confirmed COVID-19, registered between May 12, 2021, and October 10, 2021, in the outpatient department of COVID-19 at Zaweit Al-Dahmani Polyclinic in Tripoli, Libya. Inclusion and exclusion criteriaThe inclusion criteria included: (1) confirmed COVID-19 diagnosis based on a positive PCR using RT-PCR; (2) age of ≥ 18 years, without sex limitation; (3) available information of thyroid function test (TFT) results, as a minimum one serum thyroid-stimulating hormone (TSH) measurement after COVID-19 analysis; and (4) also, non-COVID-19 patients with regular thyroid function have been recruited as a control group. Exclusion criteria covered: (1) previous clinical records of TD; (2) pregnancy; and (3) patients with incomplete records for key parameters. Data collectionDemographic information of the covered patients had been collected from the laboratory scientific data. Preselected demographics and also some comorbidities of interest such as age, sex, and records of diabetes or hypertension were recorded. All COVID-19 patients were diagnosed according to presenting clinical and/or radiologic findings of COVID-19 and followed by a positive RT-PCR test for COVID-19. Laboratory testsLaboratory tests were conducted at the laboratory of Zaweit Al-Dahmani Polyclinic. Serum FT3, FT4, T3, T4, and TSH levels were tested using a Cobas e 411 analyzer® by Roche Electrochemiluminescence technology for immunoassay analysis. A complete blood count was done using the Sysmex XP-300™ Automated Hematology Analyzer. CRP, lactate dehydrogenase (LDH), liver function tests such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), direct bilirubin (DBIL), and renal function tests such as urea, creatinine (CRE), and uric acid (UA) were measured using the Roche Cobas Integra 400 plus analyzer. Evaluation of the TH profileTH results obtained from patients were classified according to the definitions given below:
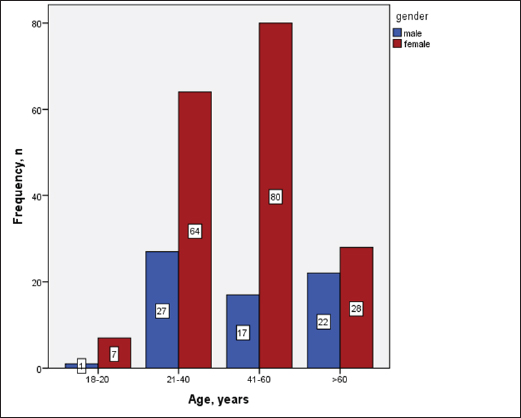
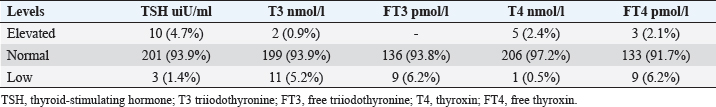
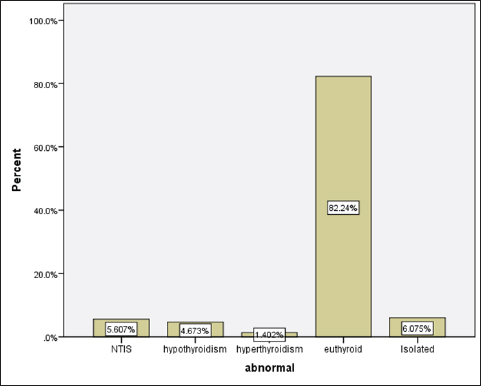
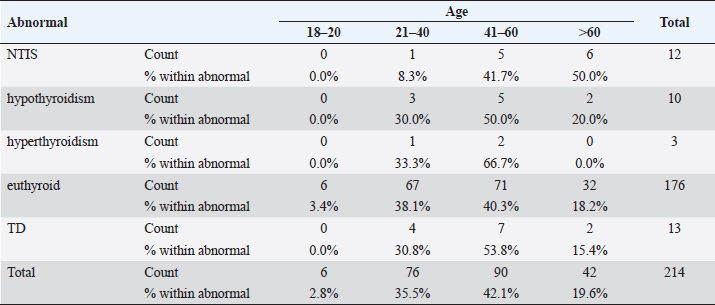
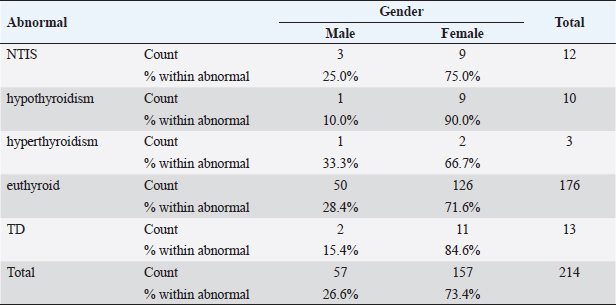
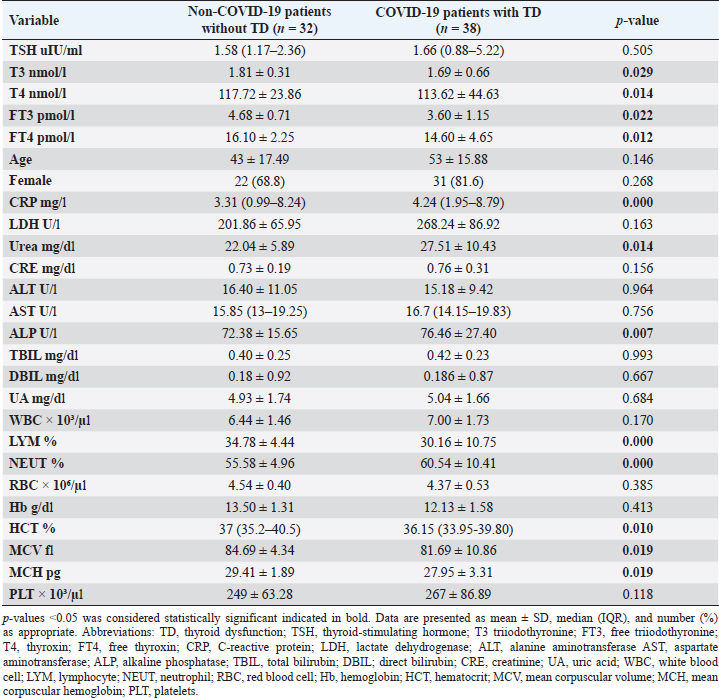
Statistical analysisStatistical analysis was performed using IBM® SPSS® version 20.0. The distribution of baseline characteristics across categories of the exposure variable was evaluated using nonparametric statistics, as determined by the one-sample Kolmogorov-Smirnov test. Continuous variables with normal distribution were presented as mean ± SD and were compared using t-tests. Variables with skewed distribution are presented as median (25th, 75th percentile) and were compared using Mann–Whitney U tests. Categorical data were presented as numbers and percentages and were compared using chi-squared tests or Fisher exact tests as appropriate. Pearson or Spearman correlations were used to assess the relationship between continuous variables as appropriate. Differences were considered statistically significant when the p-value was <0.05. Ethical approvalNot needed, as this was a retrospective study. ResultsA total number of 246 patients were included in this study; (214 COVID-19 patients and 32 non-COVID-19 patients), and the majority of the patients were females 179 (72.8%). Age of patients ranged between 18 and 88 years old, with a mean age of 46.3 ± 16.5 years (Fig. 1). Information about comorbidities was available in only 55 patients as follows: diabetes was present in 42 patients (76%), hypertension in 5 patients (9%), and diabetes with hypertension in 8 patients (15%). Concerning the thyroid status, TSH was measured in all 214 patients with COVID-19, T3, and T4 in 212 patients (99.1%), while FT3, and FT4 in 145 patients (67.8%). Of some of the overall 214 patients with COVID-19, 38 patients (17.8%) showed one or greater abnormalities in thyroid function. The commonest abnormality in TFT was low FT3 and FT4 which were discovered in 9 patients (6.2%). The distribution of altered thyroid function parameters is given in Table 1. Abnormal TFTs were observed in 38 patients (17.8%) of them, 12 patients (5.6%) were NTIS, followed by 10 patients (4.7%) with hypothyroidism, of those, 4 patients had overt hypothyroidism, 3 patients had subclinical hypothyroidism, 2 patients had high TSH concentration with normal T3 and T4 concentration, and one patient had high TSH concentration with low T3 concentration and normal T4 concentration (FT3 and FT4 were missed). Hyperthyroidism was observed in 3 patients (1.4%). Of these, one patient had subclinical hyperthyroidism, while 2 patients had low TSH with high/or normal T4 and normal T3 concentrations (FT3 and FT4 were missed). One abnormality in thyroid function was observed in 13 (6.1%) patients including isolated high T3, T4, and FT4 in 2, 4, and 2 patients, respectively, and isolated mildly low FT4 in 5 patients (Fig. 2). TH abnormalities according to age and gender are presented in Tables 2 and 3. The demographic and laboratory results of the covered non-COVID-19 patients without TD as a control group and COVID-19 patients with TD are provided in Table 4. The mean age of non-COVID-19 patients without TD, and COVID-19 patients with TD was 43.25 ± 17.49 years, and 52.95 ± 15.88 years, respectively (p =0.146). In both groups, most of the patients were females (68.8% and 81.6%, respectively; p =0.268). The laboratory results showed that COVID-19 patients with TD had higher CRP (p=0.000), urea (p=0.014), alkaline phosphatase (ALP) (p=0.007), neutrophil (p=0.000), and had lower lymphocyte (p=0.000), lower hematocrit (HCT) (p=0.010), lower mean corpuscular volume (MCV) (p=0.019), lower mean corpuscular hemoglobin (MCH) (p=0.019) when compared to non-COVID-19 patients without TD.
Fig. 1. Age and sex distribution of the study patients (N=246). Table 1. Distribution of various TFT values of COVID-19 patients in three groups (high, regular, low) together with their percent inside brackets n=214.
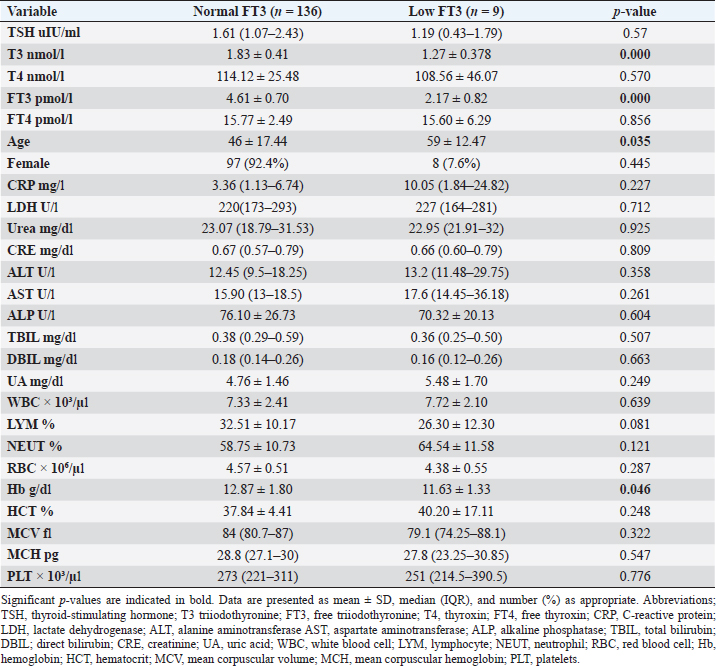
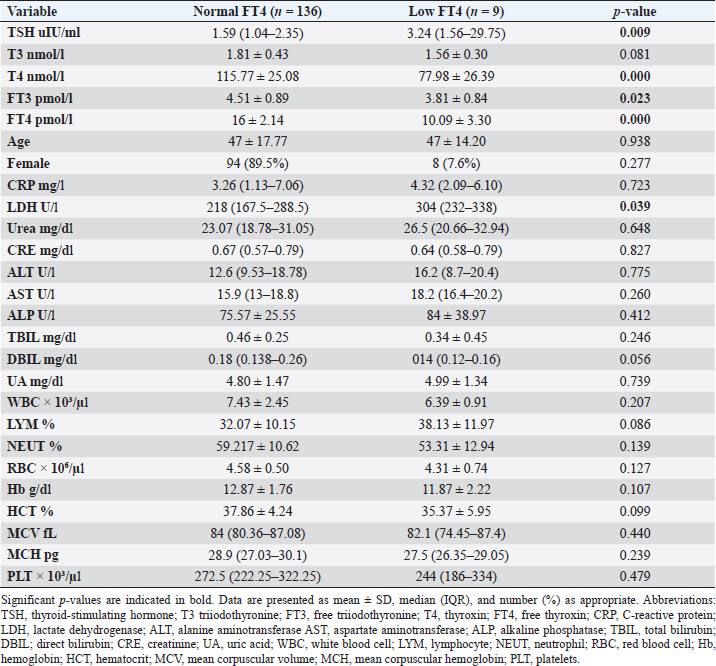
Fig. 2. TH abnormalities in COVID-19 patients (n=214). Comparison between COVID-19 patients with normal FT3 and low FT3When comparing COVID-19 patients with normal FT3 with those having low FT3 as shown in Table 5, patients with low FT3 additionally had lower T3 (p=0.000) than those with normal FT3, and there has been no vast difference in the TSH (p=0.57), T4 (p=0.570), and FT4 (p=0.856) among patients with normal FT3 and low FT3. regarding demographic characteristics, FT3 was a significant age difference (p=0.0.5), patients with low FT3 have been drastically older as compared to patients with regular FT3, even as there was no sizable difference in FT3 according to sex (p=0.445) between patients with regular FT3 and low FT3. The laboratory results confirmed that COVID-19 patients with low FT3 had drastically lower hemoglobin (Hb) (p=0.046). Comparison between COVID-19 patients with normal FT4 and low FT4The comparison between COVID-19 patients with normal FT4 and those having low FT4 is presented in Table 6. Patients with low FT4 had higher TSH as expected (p=0.009), lower T4 (p=0.000), and FT3 (p=0.023) than those with normal FT4. Regarding demographic characteristics, there was no significant difference in FT4 according to age (p=0.938), and sex (p=0.277) between patients with normal and low FT4. The laboratory results showed that COVID-19 patients with low FT4 had significantly higher LDH (p=0.039). Correlation of descriptive biochemical markers in all patients with thyroid profileTHs were shown to have a longitudinal relationship with other baseline hematological, some inflammatory biomarkers, and biochemical markers (Table 7). Table 2. TH abnormalities with age among COVID-19 patients.
Table 3. TH abnormalities according to gender among COVID-19 patients.
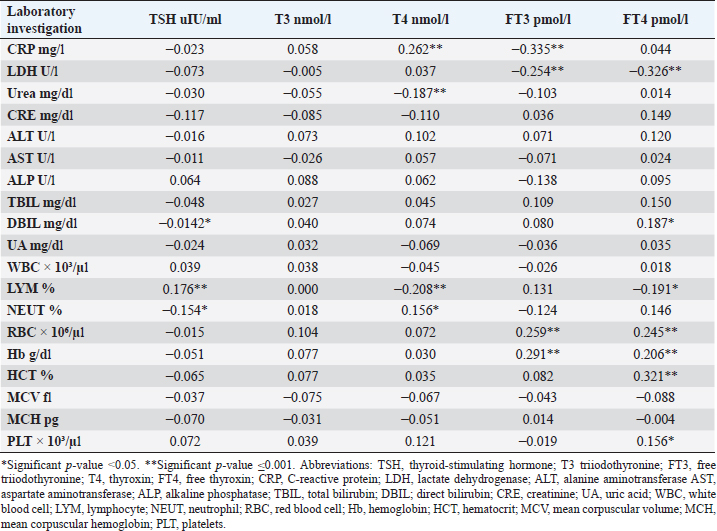
CRP was positively correlated with T4 (r=0.262, p < 0.001), while negatively correlated with FT3 (r=−0.335, p < 0.001). LDH was found to be negatively correlated with both FT3 (r=−0.254, p < 0.001) and FT4 (r=−0.326, p < 0.001). Urea was shown to be negatively correlated with T4 (r=−0.187, p < 0.001). DBIL was negatively correlated with TSH (r=−0.142, p < 0.05), while positively correlated with FT4 (r=0.187, p < 0.05). Lymphocytes were found to be positively correlated with TSH (r=0.176, p < 0.001), while negatively correlated with both T4 (r=−0.208, p < 0.001) and FT4 (r=−0.191, p < 0.001). Neutrophil count was negatively correlated with TSH (r=−0.154, p < 0.05), while positively correlated with T4 (r=0.156, p < 0.05). Furthermore, red blood cells (RBC) were positively correlated with both FT3 (r=0.259, p < 0.001) and FT4 (r=0.245, p < 0.001), Also Hb was positively correlated with both FT3 (r=0.291, p < 0.001) and FT4 (r=0.206, p < 0.001), whereas HCT and platelets were positively correlated with FT4 (r=0.321, p < 0.001), (r=0.156, p < 0.05), respectively. DiscussionIn this study, most COVID-19 patients were euthyroid (82.24%), normal range of TSH levels was observed in (93.9%) of COVID-19 patients, with TD being reported in 17.8%, this is consistent with the results obtained by other studies (Giovanella et al., 2021; Lui et al., 2021b). Giovanella et al. (2021) demonstrated that the prevalence of TD in COVID-19 patients largely varies between 13% and 64% of COVID-19 patients. Table 4. Comparison between non-COVID-19 patients without thyroid dysfunction, and COVID-19 patients with thyroid dysfunction.
Among outpatients with COVID-19 infection; 12 patients had NTIS, 10 had hypothyroidism, 3 had hyperthyroidism, and in another 13 patients at least one abnormality in thyroid function was detected (isolated THs abnormalities). The results of this study showed that (12/214, 5.6%) of COVID-19 patients had decreased total T3 and/or FT3 levels with normal total and FT4 levels and normal TSH levels observed, suggesting the presence of a component of NTIS. Also, one of them had low FT3 and high FT4 levels, showing that this form could be a combination of NTI and thyrotoxicosis, in line with the previous report by Chen et al. (2021), Khoo et al. (2021), and Lui et al. (2021b). In severe illness, both acute and chronic NTIS can arise in euthyroid patients. The preliminary position of NTIS appears to be para-physiological, adaptive, and protective, which lowers metabolic expenditure. despite the fact that it can be harmful and slow down the recovery process like different homeostatic responses of the body (Fliers et al., 2015). Table 5. Comparison between COVID-19 patients with normal FT3 and low FT3.
This research verified that low FT3 and occasionally, a reduction of TSH levels in COVID-19 patients, defined as NTIS, may be explained either by targeting the thyroid gland by SARS-CoV-2 through access to the follicular thyroid cells that express the ACE-2 receptor as SARS-CoV-2 cell access receptor (Rotondi et al., 2021) or particular and direct harm to the pituitary TSH-secreting cells by the SARS-CoV-2 (Chen et al., 2021). The results of this study indicated that COVID-19 patients with low FT3 were significantly older, also FT3 levels were negatively correlated with higher levels of inflammatory markers including CRP, and LDH, in agreement with the results of other studies (Zou et al., 2020; Güven and Gültekin, 2021; Lui et al., 2021a, 2021b;). These findings might reflect that low FT3, whether FT4 and TSH were normal or reduced, may have prognostic relevance as it was associated with worsening clinical severity of COVID-19 (Gao et al., 2021). The current study also found that 4.7% (10/214) of COVID-19 patients had hypothyroidism, approximately 50% of patients were around 50 years and older, and more than two-thirds were females. These results follow the THYROCOV study conducted in Italy which found that 5.2% (15/287) of non-ICU COVID-19 patients had hypothyroidism (Lania et al., 2020). Our results also agree with the results of a study of COVID-19 patients in Iran, where hypothyroidism was detected in 5.4% (21/390) of hospitalized patints with COVID-19 and approximately 90% of patients were 50 years or older (Daraei et al., 2020), while another study found the frequency of hypothyroidism was 3.2% in ICU COVID-19 patients, the lower rate was likely to expand exclusion standards (patients with the previously recognized pituitary disorder, diabetes mellitus, kidney, and liver disease had been excluded in the study) (Güven and Gültekin, 2021). Table 6. Comparison between COVID-19 patients with normal FT4 and low FT4.
In the study by Lania et al. (2020), hypothyroidism was subclinical in 13 patients and overt in the remaining two non-ICU patients with COVID-19, whereas, Daraei et al. (2020) study found hypothyroidism was subclinical in one ICU patient and overt in the three ICU COVID-19, patients. Among the outpatients, our study found hypothyroidism was subclinical in six patients and overt in four patients with COVID-19. These results may reflect the effects of SARS-CoV-2 on the thyroid gland that may induce hypothyroidism seen mainly as subclinical (Jaiswal et al., 2021). Noteworthy, the superiority of hyperthyroidism became low at 1.4% (3/214) in this study, suppressed TSH values have been discovered in three outpatients; none of these patients had low overall FT3 values, and certainly one of them, T4 values have been above the reference range suggesting thyrotoxicosis rather than NTI. In comparison, the findings of the study by Lania et al. (2020), reported thyrotoxicosis in approximately 20% of 287 COVID-19 patients and discovered an inverse correlation between serum TSH values and IL-6 levels, excessive mortality rate, and longer hospitalization. In another retrospective study of 50 patients, TSH was reduced in about 58% of COVID-19 patients, and an inverse link between serum TSH and T3 values with the severity of COVID-19 (Chen et al., 2021). Table 7. Correlation of descriptive biochemical markers in all patients with thyroid profile (N=246).
Hyperthyroidism was not common in this study which could be due to the clinical setting where most of the patients in our study were outpatients and the possibility of thyrotoxicosis may develop during or after the resolution of COVID-19 infection (Brancatella et al., 2020; Ippolito et al., 2020; Lania et al., 2020; Ruggeri et al., 2021) and difficult follow-up of these patients after their full recovery owing to the retrospective nature of this study. Few studies have reported an association between TD and inflammatory markers, however very few have reported an association with different hematological and biochemical markers. Wang et al. (2021) confirmed that the TD group had lower counts of lymphocytes and higher leukocytes, neutrophils, CRP, and procalcitonin. They described TD as any abnormality in T3, T4, or TSH. Those findings, with this study finding, confirmed that COVID-19 patients with TD showed a statistically sizable reduction in lymphocytes and increased neutrophil, CRP, urea, and ALP in comparison to euthyroid control indicating that systemic infection might also affect deiodinase activity. Systemic inflammation; (represented through higher inflammatory markers), which is additionally associated with systemic tissue damage, ends in reduced deiodinase activity (Ilera et al., 2021). It has been reported that liver and kidney function abnormalities in COVID-19 patients either due to direct viral effect, indirect SARS-CoV-2 effect through abnormal systemic inflammatory-immune responses, or antiviral drugs that may have a toxic effect (Ciaccio and Agnello, 2020; Hwaiz et al., 2021). Our study found a weak correlation between TFT and other biochemical markers; TSH was negative and total FT4 was positively correlated with high serum DBIL concentration, whereas a weak inverse correlation was found between T4 and urea. In contrast, the study by Asghar et al. (2021) in the analysis of 54 COVID-19 patients, found a direct correlation between TSH and urea, and a nonsignificant correlation between T4 and urea, whereas serum DBIL wasn’t assessed. An inverse correlation has been observed between T4 levels and serum DBIL (Burmeister, 1995). Currently, it is still unknown what mechanisms are precisely responsible for the association of TFT parameters and the biochemical markers and how both TSH and THs would possibly result in an increase in liver function. but, for hyperthyroidism and hypothyroidism, numerous pathways were proposed. It has been shown that as much as 72% of patients with hyperthyroidism and presumably ordinary liver function may also have an elevation of at least one hepatic enzyme, ALP elevations are most commonly found (Bal and Chawla, 2010). THs metabolism is influenced by factors aside from the thyroidal state. It has been reported that T4 became negatively related to growing serum bilirubin concentration. Inhibited transport of T4 in hepatocytes patients with elevated bilirubin has been shown in severe cases of COVID-19 (Burmeister, 1995). Similarly, FT4 can be increased when overall T4 is normal or low in hyperthyroidism because of a decreased concentration of TH-binding proteins (Bal and Chawla, 2010). Moreover, previous studies found a negative correlation between T4 and urea. It has been observed that regulation of nitrogen metabolism and urea biosynthesis was increased in isolated hepatocytes of hypothyroid rats PORTALES (Marti et al., 1988). Furthermore, it has been shown that patients with uremia had low T3 and T4 before hemodialysis, and had normal T3 and T4 after hemodialysis (Shamsadini et al., 2006). These findings might suggest that THs increase catabolic activities and lead to increasing CRE and BUN concentration, which in turn switch off THs secretion by a feedback mechanism (Marti et al., 1988; Shamsadini et al., 2006). Our study also showed that TD was slightly associated with lower hematological parameters. Given the small difference in HCT, MCV, and MCH levels among TD groups and a weak positive correlation with RBC, and Hb in all patients with thyroid profiles, the contribution of thyroid abnormalities to low Hb levels or anemia may be small. It remains to be assessed in a larger randomized controlled study to further clarify whether these results are considered clinically relevant and whether these should influence practice. Our study had numerous boundaries that could cause possible bias First, the data collection was retrospective with an exceptionally small sample size. Second, records about comorbidity and medication data were inaccessible to most patients. Eventually, lacked information on antibodies for thyroid autoimmunity. ConclusionIn conclusion, in this study of outpatients with COVID-19 infection, alteration of thyroid characteristics was observed in 17.8% of the outpatients. Low FT3 and FT4 are the most common abnormalities seen in 6.2% of patients. Low FT3 is often seen in isolation, suggestive of NTIS. Low FT3 was associated with older age, and higher markers of infection consist of CRP and LDH. Both TSH and THs (T3, T4, and free fractions) correlated with a few biomarkers. Clinicians ought to be vigilant about the possible presence of thyroid function abnormalities among COVID-19 patients, particularly elderly patients, and those with elevated inflammatory markers. Conflict of interest The authors declare that there is no conflict of interest. ReferencesAloisio, E., Chibireva, M., Serafini, L., Pasqualetti, S., Falvella, F.S., Dolci, A. and Panteghini, M. (2020). A comprehensive appraisal of laboratory biochemistry tests as major predictors of COVID-19 severity. Arch. Pathol. Lab. Med. 144(12), 1457–1464; doi:10.5858/arpa.2020-0389-SA An, P.J., Zhu, Y.Z. and Yang, L.P. (2020). Biochemical indicators of coronavirus disease 2019 exacerbation and the clinical implications. Pharmacol. Res. 159, 104946; doi:10.1016/j.phrs.2020.104946 Asghar, M.S., Yasmin, F., Dapke, K., Phadke, R., Shah, S.M.I. and Bin Zafar, M.D. (2021). Derangements of biochemical markers and thyroid function analysis among COVID-19-positive patients: a developing country single-center experience. J. Med. Virol. 93(10), 5712–5717; doi:10.1002/jmv.27174 Bal, C. and Chawla, M. (2010). Hyperthyroidism and jaundice. Indian. J. Nucl. Med. IJNM. Off. J. Soc. Nucl. Med. India. 25(4), 131. Brancatella, A., Ricci, D., Viola, N., Sgrò, D., Santini, F. and Latrofa, F. (2020). Subacute thyroiditis after SARS-CoV-2 infection. J. Clin. Endocrinol. Metab. 105(7), 2367–2370. Burmeister, L.A. (1995). Reverse T3 does not reliably differentiate hypothyroid sick syndrome from euthyroid sick syndrome. Thyroid 5(6), 435–441. Chen, M., Zhou, W. and Xu, W. (2021). Thyroid function analysis in 50 patients with COVID-19: a retrospective study. Thyroid 31(1), 8–11. Ciaccio, M. and Agnello, L. (2020). Biochemical biomarkers alterations in coronavirus disease 2019 (COVID-19). Diagnosis (Berl) 7(4), 365–372; doi:10.1515/dx-2020-0057 Daraei, M., Hasibi, M., Abdollahi, H., Mirabdolhagh Hazaveh, M., Zebaradst, J., Hajinoori, M. and Asadollahi-Amin, A. (2020). Possible role of hypothyroidism in the prognosis of COVID-19. Int. Med. J. 50(11), 1410–1412. Elhadi, M., Momen, A.A. and Ali Senussi Abdulhadi, O.M. (2020). A COVID-19 case in Libya acquired in Saudi Arabia. Travel. Med. Infect. Dis. 37, 101705; doi:10.1016/j.tmaid.2020.101705 Fliers, E., Bianco, A.C., Langouche, L. and Boelen, A. (2015). Thyroid function in critically ill patients. Lancet. Diabetes. Endocrinol. 3(10), 816–825. Gao, W., Guo, W., Guo, Y., Shi, M., Dong, G., Wang, G.A., Ge, Q., Zhu, J. and Zhou, X. (2021). Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J. Endocrinol. Invest. 44(5), 1031–1040. Giovanella, L., Ruggeri, R.M., Ovčariček, P.P., Campenni, A., Treglia, G. and Deandreis, D. (2021). Prevalence of thyroid dysfunction in patients with COVID-19: a systematic review. Clin. Transl. Imaging. 9(3), 233–240. Güven, M. and Gültekin, H. (2021). The prognostic impact of thyroid disorders on the clinical severity of COVID-19: results of single-centre pandemic hospital. Int. J. Clin. Pract. 75(6), e14129. Hwaiz, R., Merza, M., Hamad, B., HamaSalih, S., Mohammed, M. and Hama, H. (2021). Evaluation of hepatic enzymes activities in COVID-19 patients. Int. Immunopharmacol. 97, 107701. Ilera, V., Delfino, L.C., Zunino, A., Glikman, P., Drnovsek, M., Reyes, A., Dios, A., Toibaro, J., Pachioli, V., Lannes, N., Guida, A. and Gauna, A. (2021). Correlation between inflammatory parameters and pituitary-thyroid axis in patients with COVID-19. Endocrine 74(3), 455–460; doi:10.1007/s12020-021-02863-2 Ippolito, S., Dentali, F. and Tanda, M. (2020). SARS-CoV-2: a potential trigger for subacute thyroiditis? Insights from a case report. J. Endocrinol. Invest. 43(8), 1171–1172. Jaiswal, P., Kumar, S., Acharya, S., Bawankule, S., Talwar, D., Dhande, R., Bagga, C., Jugtap, G., Verma, P., Patel, M. and Khan, S. (2021). Impact of COVID 19 manifestation on thyroid function status in previously euthyroid patients: a cross sectional study. Med. Sci. 25(114), 1821–1826. Khoo, B., Tan, T., Clarke, S.A., Mills, E.G., Patel, B., Modi, M., Phylactou, M., Eng, P.C., Thurston, L., Alexander, E.C., Dhillo, W.S. and Meeran, K. (2021). Thyroid function before, during, and after COVID-19. J. Clin. Endocrinol. Metab. 106(2), e803–e811; doi:10.1210/clinem/dgaa830 Lania, A., Sandri, M.T., Cellini, M., Mirani, M., Lavezzi, E. and Mazziotti, G. (2020). Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur. J. Endocrinol. 183(4), 381–387. Lui, D.T.W., Lee, C.H., Chow, W.S., Lee, A.C. H., Tam, A.R., Fong, C.H.Y., Law, C.Y., Leung, E.K.H., To, K.K.W., Tan, K.C.B. and Woo, Y.C. (2021a). Role of non-thyroidal illness syndrome in predicting adverse outcomes in COVID-19 patients predominantly of mild-to-moderate severity. Clin. Endocrinol. 95(3), 469–477. Lui, D.T.W., Lee, C.H., Chow, W.S., Lee, A.C.H., Tam, A.R., Fong, C.H.Y., Law, C.Y., Leung, E.K.H., To, K.K.W., Tan, K.C.B. and Woo, Y.C. (2021b). Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J. Clin. Endocrinol. Metab. 106(2), e926–e935. Marti, J., Portoles, M., Jimenez-Nacher, I., Cabo, J. and Jorda, A. (1988). Effect of thyroid hormones on urea biosynthesis and related processes in rat liver. Endocrinology 123(5), 2167–2174. Rotondi, M., Coperchini, F., Ricci, G., Denegri, M., Croce, L., Ngnitejeu, S.T., Villani, L., Magri, F., Latrofa, F. and Chiovato, L. (2021). Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J. Endocrinol. Invest. 44(5), 1085–1090. Ruggeri, R.M., Campennì, A., Siracusa, M., Frazzetto, G. and Gullo, D. (2021). Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones 20(1), 219–221. Scappaticcio, L., Pitoia, F., Esposito, K., Piccardo, A. and Trimboli, P. (2020). Impact of COVID-19 on the thyroid gland: an update. Rev. Endocr. Metab. Disord. 22(4), 803–815. Shamsadini, S., Darvish, M.S., Abdollahi, H., Fekri, A. and Ebrahimi, H. (2006). Creatinine, blood urea nitrogen and thyroid hormone levels before and after haemodialysis. EMHJ-East. Mediterr. Health. J. 12(1–2), 231–235. Wang, W., Su, X., Ding, Y., Fan, W., Zhou, W., Su, J., Chen, Z., Zhao, H., Xu, K., Ni, Q. and Xu, X. (2021). Thyroid function abnormalities in COVID-19 patients. Front. Endocrinol. 11, 623792. Zou, R., Wu, C., Zhang, S., Wang, G., Zhang, Q., Yu, B., Wu, Y., Dong, H., Wu, G., Wu, S. and Zhong, Y. (2020). Euthyroid sick syndrome in patients with COVID-19. Front. Endocrinol. 11, 798. | ||
| How to Cite this Article |
| Pubmed Style Ben-omar MY, Rayes AA, Alkmishi NS, Elaswad FA, Ben-younes HA, Bin-ghisheer MS, Fadli IA, Dayhum AS, Eldaghayes IM. Assessment of thyroid function and biochemical markers among COVID-19 Libyan patients. J Microbiol Infect Dis. 2023; 13(3): 128-138. doi:10.5455/JMID.2023.v13.i3.5 Web Style Ben-omar MY, Rayes AA, Alkmishi NS, Elaswad FA, Ben-younes HA, Bin-ghisheer MS, Fadli IA, Dayhum AS, Eldaghayes IM. Assessment of thyroid function and biochemical markers among COVID-19 Libyan patients. https://www.jmidonline.org/?mno=163690 [Access: January 25, 2026]. doi:10.5455/JMID.2023.v13.i3.5 AMA (American Medical Association) Style Ben-omar MY, Rayes AA, Alkmishi NS, Elaswad FA, Ben-younes HA, Bin-ghisheer MS, Fadli IA, Dayhum AS, Eldaghayes IM. Assessment of thyroid function and biochemical markers among COVID-19 Libyan patients. J Microbiol Infect Dis. 2023; 13(3): 128-138. doi:10.5455/JMID.2023.v13.i3.5 Vancouver/ICMJE Style Ben-omar MY, Rayes AA, Alkmishi NS, Elaswad FA, Ben-younes HA, Bin-ghisheer MS, Fadli IA, Dayhum AS, Eldaghayes IM. Assessment of thyroid function and biochemical markers among COVID-19 Libyan patients. J Microbiol Infect Dis. (2023), [cited January 25, 2026]; 13(3): 128-138. doi:10.5455/JMID.2023.v13.i3.5 Harvard Style Ben-omar, M. Y., Rayes, . A. A., Alkmishi, . N. S., Elaswad, . F. A., Ben-younes, . H. A., Bin-ghisheer, . M. S., Fadli, . I. A., Dayhum, . A. S. & Eldaghayes, . I. M. (2023) Assessment of thyroid function and biochemical markers among COVID-19 Libyan patients. J Microbiol Infect Dis, 13 (3), 128-138. doi:10.5455/JMID.2023.v13.i3.5 Turabian Style Ben-omar, Mouna Y., Abdunnabi A. Rayes, Nagat S. Alkmishi, Firuz A. Elaswad, Hanadi A. Ben-younes, Munay S. Bin-ghisheer, Ibtehal A. Fadli, Abdunaser S. Dayhum, and Ibrahim M. Eldaghayes. 2023. Assessment of thyroid function and biochemical markers among COVID-19 Libyan patients. Journal of Microbiology and Infectious Diseases, 13 (3), 128-138. doi:10.5455/JMID.2023.v13.i3.5 Chicago Style Ben-omar, Mouna Y., Abdunnabi A. Rayes, Nagat S. Alkmishi, Firuz A. Elaswad, Hanadi A. Ben-younes, Munay S. Bin-ghisheer, Ibtehal A. Fadli, Abdunaser S. Dayhum, and Ibrahim M. Eldaghayes. "Assessment of thyroid function and biochemical markers among COVID-19 Libyan patients." Journal of Microbiology and Infectious Diseases 13 (2023), 128-138. doi:10.5455/JMID.2023.v13.i3.5 MLA (The Modern Language Association) Style Ben-omar, Mouna Y., Abdunnabi A. Rayes, Nagat S. Alkmishi, Firuz A. Elaswad, Hanadi A. Ben-younes, Munay S. Bin-ghisheer, Ibtehal A. Fadli, Abdunaser S. Dayhum, and Ibrahim M. Eldaghayes. "Assessment of thyroid function and biochemical markers among COVID-19 Libyan patients." Journal of Microbiology and Infectious Diseases 13.3 (2023), 128-138. Print. doi:10.5455/JMID.2023.v13.i3.5 APA (American Psychological Association) Style Ben-omar, M. Y., Rayes, . A. A., Alkmishi, . N. S., Elaswad, . F. A., Ben-younes, . H. A., Bin-ghisheer, . M. S., Fadli, . I. A., Dayhum, . A. S. & Eldaghayes, . I. M. (2023) Assessment of thyroid function and biochemical markers among COVID-19 Libyan patients. Journal of Microbiology and Infectious Diseases, 13 (3), 128-138. doi:10.5455/JMID.2023.v13.i3.5 |