| Research Article | ||
J. Microbiol. Infect. Dis., (2024), Vol. 14(1): 1–7 Original Research The interplay of stress and salivary cortisol saliva in multibacillary leprosy: A cross-sectional analysis in South Sulawesi, IndonesiaSitti Musafirah Arif1* and Andi Tenri Padad21Dermatology and Venereology Department, Faculty of Medicine and Health Sciences, Universitas Muhammadiyah, Makassar, Indonesia 2Psychiatric Department, Faculty of Medicine and Health Sciences, Universitas Muhammadiyah Makassar, Indonesia *Corresponding Author: Sitti Musafirah Arif. Dermatology and Venereology Department, Faculty of Medicine and Health Sciences, Universitas Muhammadiyah, Makassar, Indonesia. Email: sitimusafirah [at] med.unismuh.ac.id Submitted: 12/01/2024 Accepted: 24/03/2024 Published: 31/03/2024 © 2024 Journal of Microbiology and Infectious Diseases
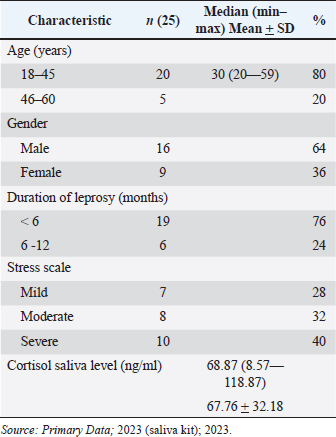
AbstractBackground: Leprosy remains a significant health concern in Indonesia, particularly in South Sulawesi, where the prevalence rate has reached 0.78 per 10,000 population. The increasing number of new cases, mainly of the multibacillary type, poses a serious epidemiological and clinical challenge. Leprosy patients often face social stigma, leading them to live in isolation, causing additional stress for the patients. Stress triggers various physiological responses, including activating the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic-adrenal-medullary system. Cortisol, the primary glucocorticoid secreted by the adrenal cortex, is the end product of the HPA axis. In the salivary glands, cortisol exists only in its active form, different from its presence in the serum. Aim: To analyze salivary cortisol levels and correlate them with the stress scale in multibacillary leprosy patients. Methods: This study used a descriptive-analytic approach with a cross-sectional method. Twenty-five samples in Makassar from November 2022 to January 2023, with the criteria of those newly diagnosed with multibacillary leprosy based on clinical and laboratory examination, participated. The stress scale was assessed with the perceived stress scale-10 (PSS-10). Using enzyme-linked immunosorbent assay to measure saliva cortisol levels. Results: The correlation for the stress scale with the cortisol saliva levels shows a negative correlation with salivary cortisol levels in this study showing a mean value of 67.76 ng/ml and a significance value of 0.000. Conclusion: There is a negative correlation between stress scale and salivary cortisol levels. The more severe the stress, the lowest cortisol saliva level. Keywords: Cortisol saliva, Stress scale, Leprosy, Multibacillary type. IntroductionLeprosy is a persistent granulomatous infection caused by Mycobacterium lepra, classified as one of the overlooked diseases that affect peripheral nerves and skin. Leprosy remains a significant health concern in Indonesia, particularly in South Sulawesi, where the prevalence rate has reached 0.78 per 10,000 population (Annual Data, 2021). However, the high number of new cases, especially of the multibacillary type, indicates a serious epidemiological problem and clinical implications, as multibacillary leprosy patients are a source of transmission and disability (Pacheco and Moraes, 2009; Shen et al., 2012; Fischer, 2017). In multibacillary leprosy, the likelihood of a leprosy reaction is greater, and disability is more likely to occur. The problems caused by disability are not only medical but also psychosocial. Leprosy patients often face social stigma, leading them to live in isolation, causing additional stress for the patients (Michgelsen, 2018; Tosepu, 2018). Stress occurs when the external environment puts pressure, conflict anxiety, and frustration on the individual (Nazli et al., 2021; Nasir et al., 2022; Susanto et al., 2023). The clinical manifestations of leprosy are largely dependent on the balance of Th1 and Th2. Tuberculoid type of leprosy is caused by a cell-mediated immune response with a Th1 pattern that limits the proliferation of the pathogen, and multibacillary type is characterized by a host with a low level of resistance to the bacilli resulting in widespread presence of the bacilli and a humoral immune response that is primarily Th2 pattern (Dabi et al., 2023). Stress is one of the processes that disrupt the balance between cellular immunity mediated by T helper1 (Th1) and humoral immunity mediated by T helper 2 (Th2), through stimulation of the HPA axis leading to the release of important stress hormones. This involves corticotropin-releasing hormone from the hypothalamus, adrenocorticotropic hormone (ACTH) from the anterior pituitary, and cortisol from the adrenal cortex. Some studies have reported a correlation between stress and cortisol levels (Terao et al., 2016; Bach et al., 2021; Krahel et al., 2021; Luo et al., 2021; Mata-Gil et al., 2021; Tanra et al., 2021; Thau et al., 2023). Cortisol, the primary glucocorticoid produced by the adrenal cortex in humans, follows a diurnal pattern of secretion influenced by pituitary-derived corticotropins, in individuals who are not experiencing stress. During inflammation, cytokines stimulate the hypothalamic-pituitary-adrenal (HPA) axis, through which glucocorticoid’s immunosuppression and anti-inflammatory effects inhibit or regulate inflammation. High levels of inflammatory cytokines can also inhibit adrenal synthesis directly (Terao et al., 2016; Gupta et al., 2018). If stimulation occurs for a long time, there will be adrenal cortex fatigue and resistance to stimulation so cortisol levels will drop. A normal serum cortisol level of >10 g/dl (276 nmol/l) implies a physiologic HPA axis (Terao et al., 2016; Dutt et al., 2022; Thau et al., 2023). In the other literature, the normal serum cortisol is 3.95–27.23 g/dl, and normal saliva cortisol is 0.5–2.16 g/dl (Indriyani et al., 2015). The difference really depends on the tool used. Approximately 95% of the cortisol released by the adrenal cortex will be bound to large proteins called corticosteroid-binding globulin and albumin to be carried throughout the body in the blood. A minor portion of unbound or free cortisol is considered to have biological activity. Due to its small molecular size and lipophilic characteristics, free cortisol will enter the cell by passive diffusion so it is possible to measure the amount of free cortisol from all body fluids including saliva (Hellhammer et al., 2009; Puhakka et al., 2020). It is mentioned that when the cortisol that diffuses to the cell membrane, reaches the acinar cells in the salivary glands, cortisol can enter the serum, but in saliva, it is only present in active form (Puhakka et al., 2020). Cortisol represents the end product of the HPA axis, which is the primary hormonal stress system in the body. It is frequently utilized as a biomarker for measuring psychological stress. Collecting cortisol from saliva is generally seen as a less intrusive method when compared to other methods such as obtaining it from serum. This ease of collection is likely a significant factor contributing to the widespread use of salivary cortisol testing. In addition, since saliva accurately reflects the biologically active portion of cortisol, it is often preferred over serum cortisol as a measurement method (Liu et al., 2017). Saliva collection can be done by drooling or using a cotton swab on the back of the tongue or palate (Indriyani et al., 2015). The salivary cortisol examination has a sensitivity of 95.3% and a specificity of 91.62%. (Kandhalu, 2013). Salivary cortisol samples can be collected in the morning at 8:00–9:00 and in the afternoon at 16:00–17:00 (Gupta et al., 2018; Almanza-Sepulveda et al., 2020; Krahel et al., 2021; Song et al., 2023). This study aims to analyze salivary cortisol levels and correlate them with the stress scale in multibacillary leprosy patients. Materials and MethodsThe research employed a descriptive, cross-sectional analytic approach. The subjects of this study amounted to 25 samples, obtained at the Makassar Skin and Health Center Hospital, South Sulawesi Province, and several health centers in the Makassar area from November 2022 to January 2023. The inclusion criteria in this study were patients aged >18 years who had been diagnosed with multibacillary leprosy based on clinical examination and were acid-fast bacilli positive and who agreed to participate in this study through informed consent. Leprosy patients who were undergoing multidrug treatment or other anti-leprosy treatment without comorbidities. Selected patients underwent history taking, physical examination, dialogue, and questionnaire filling and were given a sterile container to collect saliva. Saliva samples were collected at 8:00 a.m. morning. Assessment of the stress scale was established relying on the Perceived Stress Scale-10 (PSS-10) form, where normal (score 0), mild (score 1–26), and severe (score >26). Skala PSS-10 is a measurement tool used to measure the level of a person’s perception of stress in their life. The scale consists of 10 questions designed to assess how often a patient has felt stressed by their illness in the past few weeks. Subjects are asked to rate on a scale of 0 (never) to 4 (very often). Interpretation of question results converted in the form of scores, scores 0–7 (normal), 8–11 (mild stress), 12–15 (moderate stress), 16–20 (severe stress), and more than 21 (extremely stress). Assessment for cortisol saliva through subjects put saliva into a sterile container that has been provided as much as 2–3 cc, at 8.00–9.00 pm, then the container containing the saliva is stored in the refrigerator with a temperature of 7–10°C. After the sample is fulfilled, the sample is measured for cortisol levels using the enzyme-linked immunosorbent assay (ELISA) method (ELISA: Elabscience® QuicKey Pro Human Cortisol ELISA Kit, with the number: E- OSEL–H0006), in Hospital Laboratory University of Hasanuddin, Makassar. All data were obtained using Microsoft Excel and SPSS Statistic applications. Data presentation includes the results of the Pearson test, presented in the form of frequency distribution tables. Ethical approvalThis study was conducted after obtaining approval from the Faculty of Medicine and Health Sciences Ethics Committee of Universitas Muhammadiyah Makassar with registration number 244/UM.PKE/Xi/44/2022. ResultsCharacteristics of the research subjectsThe study included 25 participants with multibacillary-type leprosy comprising 16 males (64%) and 9 females (36%), with varying ages included in the range of 18–45 years old. The characteristic can be seen in Table 1. Characteristic of cortisol saliva levelsCharacteristic of cortisol saliva levels lower in those who had leprosy for less than 6 months and also in those who experienced severe stress, as we see in Table 2. Correlation of stress scale with cortisol saliva levelThe correlation analysis was performed using the Spearman correlation method because the stress scale data is not normally distributed based on the Saphiro-Wilk test. The statistical analysis shows a significant correlation (p=0.00) between stress scale and cortisol saliva in the multibacillary type of leprosy. The result can be seen in Table 3. This result can also be seen in Figure 1, we can see the relationship between the stress scale and salivary cortisol levels. The “r” (rho) value in this context is which signifies a very strong negative monotonic relationship between stress and salivary cortisol. The closer this value is to a maximum value of 1 (−0.933 in this case), the stronger the relationship between the two variables. DiscussionMen constituted the majority of subjects in this study at 64%, while women accounted for 36%. This aligns with an earlier investigation conducted by Prakoeswa et al. (2022) which states that in most countries in Asia. Leprosy is more prevalent among males, because it is associated with several factors, such as differences in behavior, and lifestyle, and also men are more active outside so they are vulnerable to infection, while women are more accustomed to taking care of themselves and maintaining health (Indrawati et al., 2022; Prakoeswa et al., 2022). Leprosy can occur at any age, but the most common incidence is in the age range of 20–30 years. Young and productive people have a greater risk of being exposed to leprosy (Pacheco et al., 2009; Prakoeswa et al., 2022). The average age of leprosy patients in this study was 30 with the highest age range being 18–45 years. Salivary cortisol levels in the subjects of this study were below normal with a mean value of 68.87 ug/ml (=0.006887 g/dl), the normal saliva level is 0.5–2.16 g/dl. This is similar to the research of Ayudianty et al. (2014) which states that serum cortisol levels in multibacillary-type leprosy patients are low, due to chronic exposure to proinflammatory cytokines (Ayudianty et al., 2014). Also, research by Nazli et al. (2021) showed low cortisol levels in all multibacillary leprosy research subjects that may be caused by adrenal insufficiency (Puhakka et al., 2020; Nazli et al., 2021). This study is different from research by Dabi et al. (2023) who showed that plasma cortisol levels in lepromatous leprosy patients (multibacillary) were higher than in healthy controls. This is due to changes in endocrine hormone levels that occur in multibacillary leprosy patients reflecting clinical and immunological conditions (Ayudianty et al., 2014; Dabi et al., 2023). Table 1. Characteristics of the research subjects.
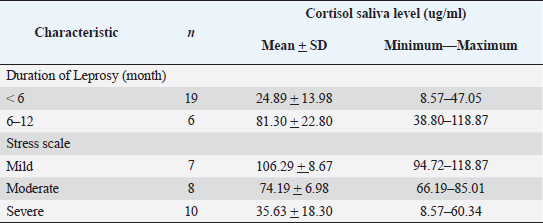
Table 2. Characteristic of cortisol saliva levels.
Concerning cortisol saliva levels in individuals with leprosy, we observed that those who had leprosy for less than 6 months had salivary cortisol levels lower compared to those who suffered for more than 6 months, with a mean value of 81.30 ng/ml. This finding aligns with Ayudianti et al.’s study which suggested that exposure to acute conditions when TNF- α production is significant and elevated, can disrupt the secretion of ACTH, leading to a decrease in serum cortisol levels. However, once the acute phase subsides, the body compensates by increasing the cortisol level due to negative feedback from the HPA axis (Ayudianty et al., 2014; Adisty et al., 2015; Nazli et al., 2021). Persistent and prolonged exposure to stressors can cause overstimulation of the HPA axis resulting in fluctuations in cortisol levels (Jones et al., 2021). Table 3. Correlation of stress scale with cortisol saliva level.
The stress scale assessment was made based on the PSS-10 form. The PSS is a widely used tool to measure how individuals perceive stress in their lives. It consists of 10 questions that individuals answer regarding their personal experiences, assessing the extent to which they perceive various life situations as stressful (Spencer et al., 2012). This assessment has also been used extensively in various studies linking cortisol levels and stress scales. The study by Walvekar et al. (2015), on the correlation between serum cortisol and stress scales in policemen, found a positive correlation between stress and serum cortisol that was statistically significant. The results of this study were different from our study which showed a negative correlation between stress scale and cortisol levels. According to the stress scale data, ten subjects encountered severe stress along with reduced cortisol levels, and among them were subjects who had leprosy for less than 6 months. This occurrence can be linked to the existing societal stigma in South Sulawesi, where leprosy is still viewed as an inherited illness, causing family members who have leprosy to face exclusion. This phenomenon puts considerable emotional pressure on those affected by leprosy, especially those newly diagnosed with leprosy, leading to stress and decreased cortisol levels.
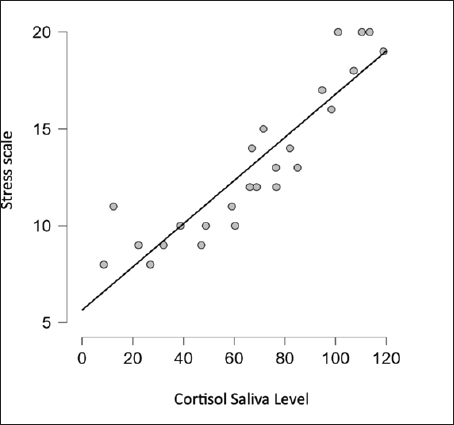
Fig. 1. Result difference cortisol level in stress scale. Cortisol is an adrenal glucocorticoid hormone secreted by the zona fasciculate in the adrenal cortex (Hellhammer et al., 2009; Terao et al., 2016; Chojnowska et al., 2021; Yuksel et al., 2023) which can regulate sugar metabolism and can be found in different bodily fluids such as saliva, urine, and serum (Chojnowska et al., 2021; Krahel et al., 2021; Song et al., 2023). In humans, it serves as the outcome of the HPA axis. The body might generate an appropriate stress reaction when confronted with stress-inducing factors (Hellhammer et al., 2009; Dutt et al., 2022; Thau et al., 2023). Cortisol holds paramount importance as a steroid hormone, exerting a substantial impact on bodily metabolism and exerting notable influence on both peripheral tissues and the central nervous system. Variations in cortisol release frequently coincide with psychiatric conditions, and the restoration of its levels correspond to enhancements in the patient’s well-being (Chojnowska et al., 2021; Krahel et al., 2021; Dutt et al., 2022; Song et al., 2023; Thau et al., 2023). Malfunctioning of the HPA axis is linked to psychological and functional issues, encompassing conditions such as anxiety, sleeplessness, depression, bipolar disorder, mood disorders, cognitive impairment, fibromyalgia (FM, irritable bowel syndrome, and alcoholism). The HPA axis responds to stressors by releasing cortisol. Salivary cortisol, utilized as an indicator of stress, has garnered significant attention in contemporary research (Spencer et al., 2012; Song et al., 2023). Increased cortisol secretion under stress impacts brain function and condition. The hippocampus, owing to its abundant steroid receptors, is the region most subjected to elevated levels of this hormone. Repeated stress leads to alterations in neuronal structure, and while transient stress-related atrophy can be reversible, chronic stress can result in the demise of hippocampus neurons (Hellhammer et al., 2009; Spencer et al., 2012; Luo et al, 2021). Thus, prolonged, recurrent, and severe stress can have adverse effects on people with leprosy including psychological and social impacts. This study has several limitations, namely that the research subjects were not compared with other types of leprosy. Future research efforts should include a wider range of variables for comparison to obtain more precise findings. ConclusionThere is a negative correlation between stress scale and salivary cortisol levels. The more severe the stress, the lowest cortisol saliva level. AcknowledgmentsSpecial thanks to the director and staff of the Skin and Health Center Hospital, the South Sulawesi Provincial Health Office, and the heads of health centers in Makassar. Thank you to Hospital Laboratory, Universitas Hasanuddin, Makassar. Thanks to Siti Khofifatul Ashlah, an undergraduate student from the Faculty of Medicine and Health Sciences, University of Muhammadiyah Makassar, who assisted in preparing this journal article based on the study report. Authors contributionsBoth authors contributed to this study. Both authors read and approved the final manuscript. Conflict of interestThe authors declare that there is no conflict of interest. FundingThe research was funded by an Internal Grant from the Universitas Muhammadiyah Makassar under the Research and Community Service Grants 2022 program. Data availabilityAll data are provided in the manuscript. Any additional data needed can be provided upon reasonable request from the corresponding author. ReferencesAlmanza-Sepulveda, M.L., Fleming, A.S. and Jonas, W. (2020). Mothering revisited: a role for cortisol? Horm. Behav. 121, 104679. Annual-Data. 2021. “Prevention of Communicable Disease Div.” South Sulawesi, Indonesia. Ayudianti, P., Suyoso, S. and Indramaya, D.M. (2014). Kadar kortisol serum pasien eritema nodosum leprosum (ENL) Baru (Serum cortisol levels in new erythema nodusum leprosum patients). Periodical. Dermatol. Venereol. 26(2), 90–96. Bach, A., Ceron, J.J., Maneja, R., Llusià, J., Penuelas, J. and Escribano, D. (2021). Evolution of human salivary stress markers during an eight-hour exposure to a mediterranean holm oak forest. A pilot study. Forests. 12(11), 1600. Chojnowska, S., Ptaszyńska-Sarosiek, I., Kępka, A., Knaś, M. and Waszkiewicz, N. (2021). Salivary biomarkers of stress, anxiety and depression. J. Clin. Med. 10(3), 517. Dabi, Y.T., Degechisa, S.T., Bobosha, K. and Wassie, L. (2023). Changes in plasma levels of endocrine hormones in lepromatous leprosy patients. IJID Regions. 6, 58–61. Fischer, M. (2017). Leprosy–an overview of clinical features, diagnosis, and treatment. J. Dtsch. Dermatol. Ges. 15(8), 801–827. Gupta, A., Sharma, P.K., Garga, U.C. and Sharma, L.K. (2018). Adrenal cortical function and adrenal volume in leprosy: a study of 40 cases. Lepr. Rev. 89(2), 148–157. Hellhammer, D.H., Wüst, S. and Kudielka, B.M. (2009). Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 34(2), 163–171. Indrawati, D., Astari, Hidayati, A.N., Damayanti, S., Utomo, B., Kusumaputra, B.H., Alinda, M.D., Prakoeswa, C.R.S. and Listiawan, M.Y. (2022). Risk factors of acute and chronic erythema nodosum leprosum in Dr. Soetomo general Academic Hospital Surabaya. Pharmacogn. J. 14(6), 766–70. Adisty, N.I., Hutomo, M. and Indramaya, D.M. (2015). Kadar kortisol saliva menggambarkan kadar kortisol serum pasien dermatitis atopik (salivary cortisol levels representing serum cortisol levels in atopic dermatitis patients). BIKKK-Berkala Ilmu Kesehatan Kulit Dan Kelamin. Periodical. Dermatol. Venereol. 27(3), 170–175. Jones, C. and Gwenin, C. (2021). Cortisol level dysregulation and its prevalence—Is it nature’s alarm clock? Physiol. Rep. 8(24), e14644. Kandhalu, P. (2013). Effects of cortisol on physical and psychological aspects of the body and effective ways by which one can reduce stress. Berkeley Sci. J. 18(1), 14–16. Krahel, A., Paszynska, E., Slopien, A., Gawriolek, M., Otulakowska-Skrzynska, J., Rzatowski, S., Hernik, A., Hanć, T., Bryl, E., Szczesniewska, P., Bilska, K., Duda, J., Tyszkiewicz-Nwafor, M. and Dmitrzak-Weglarz, M. (2021). Stress/immune biomarkers in saliva among children with ADHD status. Int J. Environ. Res. Public. Health. 18(2), 769. Liu, J.J., Ein, N., Peck, K., Huang, V., Pruessner, J.C. and Vickers, K. (2017). Sex differences in salivary cortisol reactivity to the trier social stress test (TSST): a meta-analysis. Psychoneuroendocrinology. 82, 26–37. Luo, Y., Kiriya, M., Tanigawa, K., Kawashima, A., Nakamura, Y., Ishii, N. and Suzuki, K. (2021). Host-related laboratory parameters for leprosy reactions. Front. Med. 8, 694376. Mata-Gil, S., Sánchez-Cabaco, A., Del Moral-Martínez, J., Seisdedos-Benito, A. and Lundberg, U. (2021). Concentrations of salivary cortisol in victims of intimate partner violence according to the CIRCORT database. Int. J. Environ. Res. Public. Health. 18(20), 10819. Dutt, M., Wehrle, C.J. and Jialal, I. (2022). Physiology, adrenal gland. [Internet]. Treasure island, FL: StatPearls Publishing. Michgelsen, J., Peters, R.M., van Brakel, W.H. and Irwanto, I. (2018). The differences in leprosy-related stigma between 30 sub- districts in Cirebon District, Indonesia. Lepr. Rev. 89(1), 65–76. Nasir, A., Yusuf, A., Listiawan, M.Y., Makhfudli, M., Muhalla, H.I., Wahyudi, A.S. and Muhith, A. (2022). Relationship between resilience, coping resources, and psychological well-being with stress of leprosy as a predictor. A correlation study through the structural equation models. Clin. Epidemiol. Glob. Health. 17, 101151. Nazli, P.A.N., Lubis, S.R. and Khairina, K. (2021). Correlation between the stress scale with cortisol levels in leprosy patients. Bali. Med. J. 10(1), 500–504. Pacheco, A.G. and Moraes, M.O. (2009). Genetic polymorphisms of infectious diseases in case-control studies. Dis. Markers. 27(3-4), 173–186. Prakoeswa, C.R.S., Lubis, R.S., Anum, Q., Argentina, F., Menaldi, S.L., Gunawan, H., Yuniati, R., Mulianto, N.R., Siswati, A.S., Widasmara, D., Rusyati, L.M.M., Mamuaja, E.H., Muchtar, V., Agusni, R.I., Kusumaputra, B.H., Alinda, M.D. and Listiawan, M.Y. (2022). Epidemiology of leprosy in Indonesia: a retrospective study. Berk Ilmu Kesehat Kulit dan Kelamin, 34(1), 29–35. Puhakka, I.J. and Peltola, M.J. (2020). Salivary cortisol reactivity to psychological stressors in infancy: a meta-analysis. Psychoneuroendocrinology. 115, 104603. Shen, J., Bathyala, N., Kroeger, A., Arana, B., Pannikar, V., Mou, H., Bao, X., Yang, R., Manickam, P., Li, W., Zhou, M. and Want, J. (2012). Bacteriological results and leprosy reactions among MB leprosy patients treated with uniform multidrug therapy in China. Lepr Rev. 83(2), 164–171. Song, M., Bai, H., Zhang, P., Zhou, X. and Ying, B. (2023). Promising applications of human-derived saliva biomarker testing in clinical diagnostics. Int. J. Oral. Sci. 15(1), 2. Spencer, J.S., Duthie, M.S., Geluk, A., Balagon, M.F., Kim, H.J., Wheat, W.H., Chatterjee, D., Jackson, M., Li, W., Kurihara, J.N., Maghanoy, A., Mallari, I., Saunderson, P., Brennan, P.J. and Dockrell, H.M. (2012). Identification of serological biomarkers of infection, disease progression and treatment efficacy for leprosy. Memórias do Instituto Oswaldo Cruz. 107, 79–89. Susanto, D.F.P., van ‘t Noordende, A.T., Septian, E.R., van Brakel, W.H., Peters, R.M.H. and Irwanto, I. (2023). The influence of leprosy and other disabilities on marital relationships and sexual health among married women in Indonesia: a qualitative study into experiences and coping. Lepr. Rev. 94(1), 19–36. Tanra, A.J., Madeali, H., Sanusi, M., Syamsuddin, S. and Lisal, S.T. (2021). Salivary alpha-amylase enzyme and salivary cortisol level in depression after treatment with fluoxetine. Open Access Maced. J. Med. Sci. 9(T3), 305–310. Terao, M. and Katayama, I. (2016). Local cortisol/corticosterone activation in skin physiology and pathology. J. Dermatol. Sci. 84(1), 11–16. Thau, L., Gandhi, J. and Sharma, S. (2023). Physiology, cortisol. Treasure Island, FL: StatPearls. Tosepu, R., Gunawan, J., Effendy, DS., Fadmi FR. (2018). Stigma and increase of leprosy cases in SouthEast Sulawesi Province, Indonesia. Afr. Health. Sci. 18(1), 29–31. Yuksel, D., Kiss, O., Prouty, D., Arra, N., Volpe, L., Baker, F.C. and de Zambotti, M. (2023). Stress, hypothalamic pituitary adrenal axis activity and autonomic nervous system function in adolescents with insomnia. Int. J. Psychophysiol. 187, 43–53. | ||
| How to Cite this Article |
| Pubmed Style SMA, Padad AT. The Interplay of Stress and Salivary Cortisol Saliva in Multibacillary Leprosy: A Cross-Sectional Analysis in South Sulawesi, Indonesia. J Microbiol Infect Dis. 2024; 14(1): 1-7. doi:10.5455/JMID.2024.v14.i1.1 Web Style SMA, Padad AT. The Interplay of Stress and Salivary Cortisol Saliva in Multibacillary Leprosy: A Cross-Sectional Analysis in South Sulawesi, Indonesia. https://www.jmidonline.org/?mno=192781 [Access: May 17, 2024]. doi:10.5455/JMID.2024.v14.i1.1 AMA (American Medical Association) Style SMA, Padad AT. The Interplay of Stress and Salivary Cortisol Saliva in Multibacillary Leprosy: A Cross-Sectional Analysis in South Sulawesi, Indonesia. J Microbiol Infect Dis. 2024; 14(1): 1-7. doi:10.5455/JMID.2024.v14.i1.1 Vancouver/ICMJE Style SMA, Padad AT. The Interplay of Stress and Salivary Cortisol Saliva in Multibacillary Leprosy: A Cross-Sectional Analysis in South Sulawesi, Indonesia. J Microbiol Infect Dis. (2024), [cited May 17, 2024]; 14(1): 1-7. doi:10.5455/JMID.2024.v14.i1.1 Harvard Style , S. M. A. & Padad, . A. T. (2024) The Interplay of Stress and Salivary Cortisol Saliva in Multibacillary Leprosy: A Cross-Sectional Analysis in South Sulawesi, Indonesia. J Microbiol Infect Dis, 14 (1), 1-7. doi:10.5455/JMID.2024.v14.i1.1 Turabian Style , Sitti Musafirah Arif, and Andi Tenri Padad. 2024. The Interplay of Stress and Salivary Cortisol Saliva in Multibacillary Leprosy: A Cross-Sectional Analysis in South Sulawesi, Indonesia. Journal of Microbiology and Infectious Diseases, 14 (1), 1-7. doi:10.5455/JMID.2024.v14.i1.1 Chicago Style , Sitti Musafirah Arif, and Andi Tenri Padad. "The Interplay of Stress and Salivary Cortisol Saliva in Multibacillary Leprosy: A Cross-Sectional Analysis in South Sulawesi, Indonesia." Journal of Microbiology and Infectious Diseases 14 (2024), 1-7. doi:10.5455/JMID.2024.v14.i1.1 MLA (The Modern Language Association) Style , Sitti Musafirah Arif, and Andi Tenri Padad. "The Interplay of Stress and Salivary Cortisol Saliva in Multibacillary Leprosy: A Cross-Sectional Analysis in South Sulawesi, Indonesia." Journal of Microbiology and Infectious Diseases 14.1 (2024), 1-7. Print. doi:10.5455/JMID.2024.v14.i1.1 APA (American Psychological Association) Style , S. M. A. & Padad, . A. T. (2024) The Interplay of Stress and Salivary Cortisol Saliva in Multibacillary Leprosy: A Cross-Sectional Analysis in South Sulawesi, Indonesia. Journal of Microbiology and Infectious Diseases, 14 (1), 1-7. doi:10.5455/JMID.2024.v14.i1.1 |